Abstract
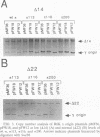
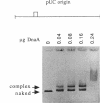
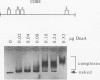
Replication of the gamma origin of Escherichia coli plasmid R6K requires pi protein, encoded by the R6K pir gene, and many host factors, including DnaA protein. Pi has dual roles, activating replication at low levels and inhibiting replication at high levels. The inhibitory function of pi is counteracted by integration host factor and a specific sequence of the origin called the enhancer. This 106-bp DNA segment contains a binding site for DnaA protein (DnaA box 1). In this study, we mutated this site to determine if it was required for the enhancer's function. Using gamma origin derivative plasmids with the DnaA box 1 altered or deleted, we show that this site is necessary to protect the origin against levels of wild-type pi protein that would otherwise inhibit replication. To show that the base substitutions in DnaA box 1 weakened the binding of DnaA, we developed a new application of the agarose gel retardation assay. This quick and easy assay has broad applicability, as shown in binding studies with DNA fragments carrying a different segment of the R6K origin, the chromosomal origin (oriC), or the pUC origin. The gel retardation assay suggests a stoichiometry of DnaA binding different from that deduced from other assays.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alazard R., Bétermier M., Chandler M. Escherichia coli integration host factor stabilizes bacteriophage Mu repressor interactions with operator DNA in vitro. Mol Microbiol. 1992 Jun;6(12):1707–1714. doi: 10.1111/j.1365-2958.1992.tb00895.x. [DOI] [PubMed] [Google Scholar]
- Braun R. E., O'Day K., Wright A. Autoregulation of the DNA replication gene dnaA in E. coli K-12. Cell. 1985 Jan;40(1):159–169. doi: 10.1016/0092-8674(85)90319-8. [DOI] [PubMed] [Google Scholar]
- Chelm B. K., Geiduschek E. P. Gel electrophoretic separation of transcription complexes: an assay for RNA polymerase selectivity and a method for promoter mapping. Nucleic Acids Res. 1979 Dec 11;7(7):1851–1867. doi: 10.1093/nar/7.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Dellis S., Filutowicz M. Integration host factor of Escherichia coli reverses the inhibition of R6K plasmid replication by pi initiator protein. J Bacteriol. 1991 Feb;173(3):1279–1286. doi: 10.1128/jb.173.3.1279-1286.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellis S., Schatz T., Rutlin K., Inman R. B., Filutowicz M. Two alternative structures can be formed by IHF protein binding to the plasmid R6K gamma origin. J Biol Chem. 1992 Dec 5;267(34):24426–24432. [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Horiuchi K., Zinder N. D. The functional origin of bacteriophage f1 DNA replication. Its signals and domains. J Mol Biol. 1984 Feb 5;172(4):507–521. doi: 10.1016/s0022-2836(84)80020-0. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Appelt K. The integration host factor of Escherichia coli binds to multiple sites at plasmid R6K gamma origin and is essential for replication. Nucleic Acids Res. 1988 May 11;16(9):3829–3843. doi: 10.1093/nar/16.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filutowicz M., Inman R. A compact nucleoprotein structure is produced by binding of Escherichia coli integration host factor (IHF) to the replication origin of plasmid R6K. J Biol Chem. 1991 Dec 15;266(35):24077–24083. [PubMed] [Google Scholar]
- Filutowicz M., McEachern M. J., Helinski D. R. Positive and negative roles of an initiator protein at an origin of replication. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9645–9649. doi: 10.1073/pnas.83.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filutowicz M., McEachern M. J., Mukhopadhyay P., Greener A., Yang S. L., Helinski D. R. DNA and protein interactions in the regulation of plasmid replication. J Cell Sci Suppl. 1987;7:15–31. doi: 10.1242/jcs.1987.supplement_7.2. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Uhlenhopp E., Helinski D. R. Binding of purified wild-type and mutant pi initiation proteins to a replication origin region of plasmid R6K. J Mol Biol. 1986 Jan 20;187(2):225–239. doi: 10.1016/0022-2836(86)90230-5. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Kornberg A. Purified dnaA protein in initiation of replication at the Escherichia coli chromosomal origin of replication. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5817–5821. doi: 10.1073/pnas.80.19.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germino J., Bastia D. Interaction of the plasmid R6K-encoded replication initiator protein with its binding sites on DNA. Cell. 1983 Aug;34(1):125–134. doi: 10.1016/0092-8674(83)90142-3. [DOI] [PubMed] [Google Scholar]
- Germino J., Bastia D. Primary structure of the replication initiation protein of plasmid R6K. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5475–5479. doi: 10.1073/pnas.79.18.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D., Zinder N. D., Horiuchi K. Integration host factor interacts with the DNA replication enhancer of filamentous phage f1. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6262–6266. doi: 10.1073/pnas.85.17.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez V., Milisavljevic V., Kahn J. D., Gennaro M. L. Sequence and structure of cmp, the replication enhancer of the Staphylococcus aureus plasmid pT181. Gene. 1993 Nov 30;134(1):93–98. doi: 10.1016/0378-1119(93)90179-7. [DOI] [PubMed] [Google Scholar]
- Inuzuka M., Helinski D. R. Requirement of a plasmid-encoded protein for replication in vitro of plasmid R6K. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5381–5385. doi: 10.1073/pnas.75.11.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S., Ray D. S. Interference between M13 and oriM13 plasmids is mediated by a replication enhancer sequence near the viral strand origin. J Mol Biol. 1984 Aug 25;177(4):685–700. doi: 10.1016/0022-2836(84)90044-5. [DOI] [PubMed] [Google Scholar]
- Kaguni J. M., Fuller R. S., Kornberg A. Enzymatic replication of E. coli chromosomal origin is bidirectional. Nature. 1982 Apr 15;296(5858):623–627. doi: 10.1038/296623a0. [DOI] [PubMed] [Google Scholar]
- Kelley W. L., Bastia D. Conformational changes induced by integration host factor at origin gamma of R6K and copy number control. J Biol Chem. 1991 Aug 25;266(24):15924–15937. [PubMed] [Google Scholar]
- Kelley W. L., Patel I., Bastia D. Structural and functional analysis of a replication enhancer: separation of the enhancer activity from origin function by mutational dissection of the replication origin gamma of plasmid R6K. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5078–5082. doi: 10.1073/pnas.89.11.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittell B. L., Helinski D. R. Iteron inhibition of plasmid RK2 replication in vitro: evidence for intermolecular coupling of replication origins as a mechanism for RK2 replication control. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1389–1393. doi: 10.1073/pnas.88.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Helinski D. R. Plasmid R6K DNA replication. II. Direct nucleotide sequence repeats are required for an active gamma-origin. J Mol Biol. 1982 Oct 15;161(1):45–56. doi: 10.1016/0022-2836(82)90277-7. [DOI] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Lane D., Prentki P., Chandler M. Use of gel retardation to analyze protein-nucleic acid interactions. Microbiol Rev. 1992 Dec;56(4):509–528. doi: 10.1128/mr.56.4.509-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAllister T. W., Kelley W. L., Miron A., Stenzel T. T., Bastia D. Replication of plasmid R6K origin gamma in vitro. Dependence on dual initiator proteins and inhibition by transcription. J Biol Chem. 1991 Aug 25;266(24):16056–16062. [PubMed] [Google Scholar]
- McEachern M. J., Bott M. A., Tooker P. A., Helinski D. R. Negative control of plasmid R6K replication: possible role of intermolecular coupling of replication origins. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7942–7946. doi: 10.1073/pnas.86.20.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern M. J., Filutowicz M., Helinski D. R. Mutations in direct repeat sequences and in a conserved sequence adjacent to the repeats result in a defective replication origin in plasmid R6K. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1480–1484. doi: 10.1073/pnas.82.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitoso de Vargas L., Kim S., Landy A. DNA looping generated by DNA bending protein IHF and the two domains of lambda integrase. Science. 1989 Jun 23;244(4911):1457–1461. doi: 10.1126/science.2544029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji P., Greener A., Filutowicz M. Identification of a novel promoter in the replication control region of plasmid R6K. J Bacteriol. 1992 Jul;174(14):4777–4782. doi: 10.1128/jb.174.14.4777-4782.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P., Filutowicz M., Helinski D. R. Replication from one of the three origins of the plasmid R6K requires coupled expression of two plasmid-encoded proteins. J Biol Chem. 1986 Jul 15;261(20):9534–9539. [PubMed] [Google Scholar]
- Rokeach L. A., Zyskind J. W. RNA terminating within the E. coli origin of replication: stringent regulation and control by DnaA protein. Cell. 1986 Aug 29;46(5):763–771. doi: 10.1016/0092-8674(86)90352-1. [DOI] [PubMed] [Google Scholar]
- Samitt C. E., Hansen F. G., Miller J. F., Schaechter M. In vivo studies of DnaA binding to the origin of replication of Escherichia coli. EMBO J. 1989 Mar;8(3):989–993. doi: 10.1002/j.1460-2075.1989.tb03462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer C., Messer W. DnaA protein/DNA interaction. Modulation of the recognition sequence. Mol Gen Genet. 1991 Apr;226(1-2):34–40. doi: 10.1007/BF00273584. [DOI] [PubMed] [Google Scholar]
- Seufert W., Dobrinski B., Lurz R., Messer W. Functionality of the dnaA protein binding site in DNA replication is orientation-dependent. J Biol Chem. 1988 Feb 25;263(6):2719–2723. [PubMed] [Google Scholar]
- Shafferman A., Flashner Y., Hertman I., Lion M. Identification and characterization of the functional alpha origin of DNA replication of the R6K plasmid and its relatedness to the R6K beta and gamma origins. Mol Gen Genet. 1987 Jun;208(1-2):263–270. doi: 10.1007/BF00330452. [DOI] [PubMed] [Google Scholar]
- Shafferman A., Helinski D. R. Structural properties of the beta origin of replication of plasmid R6K. J Biol Chem. 1983 Apr 10;258(7):4083–4090. [PubMed] [Google Scholar]
- Shafferman A., Kolter R., Stalker D., Helinski D. R. Plasmid R6K DNA replication. III. Regulatory properties of the pi initiation protein. J Mol Biol. 1982 Oct 15;161(1):57–76. doi: 10.1016/0022-2836(82)90278-9. [DOI] [PubMed] [Google Scholar]
- Shon M., Germino J., Bastia D. The nucleotide sequence of the replication origin beta of the plasmid R6K. J Biol Chem. 1982 Nov 25;257(22):13823–13827. [PubMed] [Google Scholar]
- Singer M., Jin D. J., Walter W. A., Gross C. A. Genetic evidence for the interaction between cluster I and cluster III rifampicin resistant mutations. J Mol Biol. 1993 May 5;231(1):1–5. doi: 10.1006/jmbi.1993.1251. [DOI] [PubMed] [Google Scholar]
- Stalker D. M., Kolter R., Helinski D. R. Nucleotide sequence of the region of an origin of replication of the antibiotic resistance plasmid R6K. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1150–1154. doi: 10.1073/pnas.76.3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker D. M., Kolter R., Helinski D. R. Plasmid R6K DNA replication. I. Complete nucleotide sequence of an autonomously replicating segment. J Mol Biol. 1982 Oct 15;161(1):33–43. doi: 10.1016/0022-2836(82)90276-5. [DOI] [PubMed] [Google Scholar]
- Stenzel T. T., MacAllister T., Bastia D. Cooperativity at a distance promoted by the combined action of two replication initiator proteins and a DNA bending protein at the replication origin of pSC101. Genes Dev. 1991 Aug;5(8):1453–1463. doi: 10.1101/gad.5.8.1453. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Selzer G. Initiation of DNA synthesis in Escherichia coli. Annu Rev Biochem. 1979;48:999–1034. doi: 10.1146/annurev.bi.48.070179.005031. [DOI] [PubMed] [Google Scholar]
- Walker S. S., Francesconi S. C., Eisenberg S. A DNA replication enhancer in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4665–4669. doi: 10.1073/pnas.87.12.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. S., Francesconi S. C., Tye B. K., Eisenberg S. The OBF1 protein and its DNA-binding site are important for the function of an autonomously replicating sequence in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Jul;9(7):2914–2921. doi: 10.1128/mcb.9.7.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Goldberg I., Filutowicz M. Roles of a 106-bp origin enhancer and Escherichia coli DnaA protein in replication of plasmid R6K. Nucleic Acids Res. 1992 Feb 25;20(4):811–817. doi: 10.1093/nar/20.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yung B. Y., Kornberg A. The dnaA initiator protein binds separate domains in the replication origin of Escherichia coli. J Biol Chem. 1989 Apr 15;264(11):6146–6150. [PubMed] [Google Scholar]