Abstract
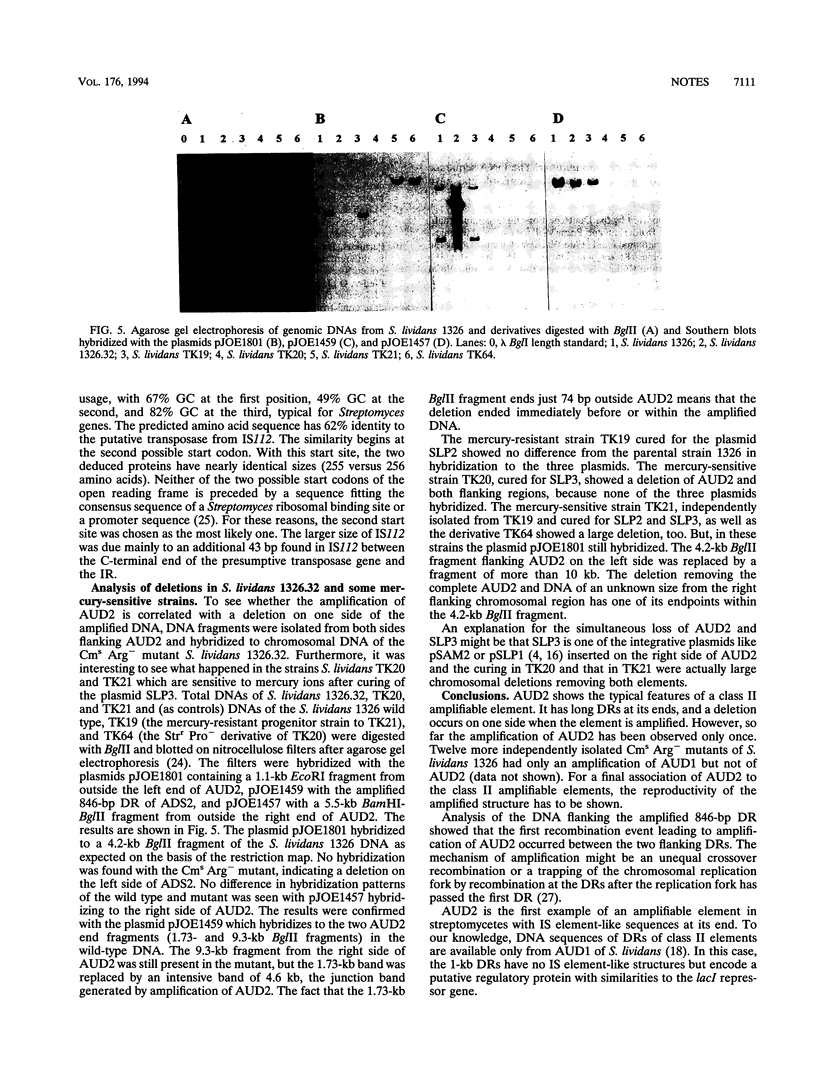
In a spontaneous, chloramphenicol-sensitive (Cms), arginine-auxotrophic (Arg-) mutant of Streptomyces lividans 1326, two amplified DNA sequences were found. One of them was the well-characterized 5.7-kb ADS1 sequence, amplified to about 300 copies per chromosome. The second one was a 92-kb sequence called ADS2. ADS2 encoding the previously isolated mercury resistance genes of S. lividans was amplified to around 20 copies per chromosome. The complete ADS2 sequence was isolated from a genomic library of the mutant S. lividans 1326.32, constructed in the phage vector lambda EMBL4. In addition, the DNA sequences flanking the corresponding amplifiable element called AUD2 in the wild-type strain were isolated by using another genomic library prepared from S. lividans 1326 DNA. Analysis of the ends of AUD2 revealed the presence of an 846-bp sequence on both sides repeated in the same orientation. Each of the direct repeats ended with 18-bp inverted repeated sequences. This insertion sequence-like structure was confirmed by the DNA sequence determined from the amplified copy of the direct repeats which demonstrated a high degree of similarity of 65% identity in nucleic acid sequence to IS112 from Streptomyces albus. The recombination event leading to the amplification of AUD2 occurred within these direct repeats, as shown by DNA sequence analysis. The amplification of AUD2 was correlated with a deletion on one side of the flanking chromosomal region beginning very near or in the amplified DNA. Strains of S. lividans like TK20 and TK21 which are mercury sensitive have completely lost AUD2 together with flanking chromosomal DNA on one or both sides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbuchner J., Cullum J. DNA amplification and an unstable arginine gene in Streptomyces lividans 66. Mol Gen Genet. 1984;195(1-2):134–138. doi: 10.1007/BF00332735. [DOI] [PubMed] [Google Scholar]
- Altenbuchner J., Cullum J. Structure of an amplifiable DNA sequence in Streptomyces lividans 66. Mol Gen Genet. 1985;201(2):192–197. doi: 10.1007/BF00425659. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Kieser T., Cohen S. N., Hopwood D. A. Excision of chromosomal DNA sequences from Streptomyces coelicolor forms a novel family of plasmids detectable in Streptomyces lividans. Mol Gen Genet. 1981;184(2):230–240. doi: 10.1007/BF00272910. [DOI] [PubMed] [Google Scholar]
- Diaz-Aroca E., Mendiola M. V., Zabala J. C., de la Cruz F. Transposition of IS91 does not generate a target duplication. J Bacteriol. 1987 Jan;169(1):442–443. doi: 10.1128/jb.169.1.442-443.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Hershberger C. L. Amplified DNA in Streptomyces fradiae. J Bacteriol. 1983 Aug;155(2):459–466. doi: 10.1128/jb.155.2.459-466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Leblond P., Demuyter P., Simonet J. M., Decaris B. Genetic instability and hypervariability in Streptomyces ambofaciens: towards an understanding of a mechanism of genome plasticity. Mol Microbiol. 1990 May;4(5):707–714. doi: 10.1111/j.1365-2958.1990.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Pernodet J. L., Simonet J. M., Guérineau M. Plasmids in different strains of Streptomyces ambofaciens: free and integrated form of plasmid pSAM2. Mol Gen Genet. 1984;198(2):35–41. doi: 10.1007/BF00328697. [DOI] [PubMed] [Google Scholar]
- Pfeifer O., Pelletier I., Altenbuchner J., van Pée K. H. Molecular cloning and sequencing of a non-haem bromoperoxidase gene from Streptomyces aureofaciens ATCC 10762. J Gen Microbiol. 1992 Jun;138(6):1123–1131. doi: 10.1099/00221287-138-6-1123. [DOI] [PubMed] [Google Scholar]
- Piendl W., Eichenseer C., Viel P., Altenbuchner J., Cullum J. Analysis of putative DNA amplification genes in the element AUD1 of Streptomyces lividans 66. Mol Gen Genet. 1994 Aug 15;244(4):439–443. doi: 10.1007/BF00286697. [DOI] [PubMed] [Google Scholar]
- Rodicio M. R., Alvarez M. A., Chater K. F. Isolation and genetic structure of IS112, an insertion sequence responsible for the inactivation of the SalI restriction-modification system of Streptomyces albus G. Mol Gen Genet. 1991 Jan;225(1):142–147. doi: 10.1007/BF00282652. [DOI] [PubMed] [Google Scholar]
- Sedlmeier R., Altenbuchner J. Cloning and DNA sequence analysis of the mercury resistance genes of Streptomyces lividans. Mol Gen Genet. 1992 Dec;236(1):76–85. doi: 10.1007/BF00279645. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Strohl W. R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992 Mar 11;20(5):961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young M., Cullum J. A plausible mechanism for large-scale chromosomal DNA amplification in streptomycetes. FEBS Lett. 1987 Feb 9;212(1):10–14. doi: 10.1016/0014-5793(87)81547-8. [DOI] [PubMed] [Google Scholar]



