Abstract
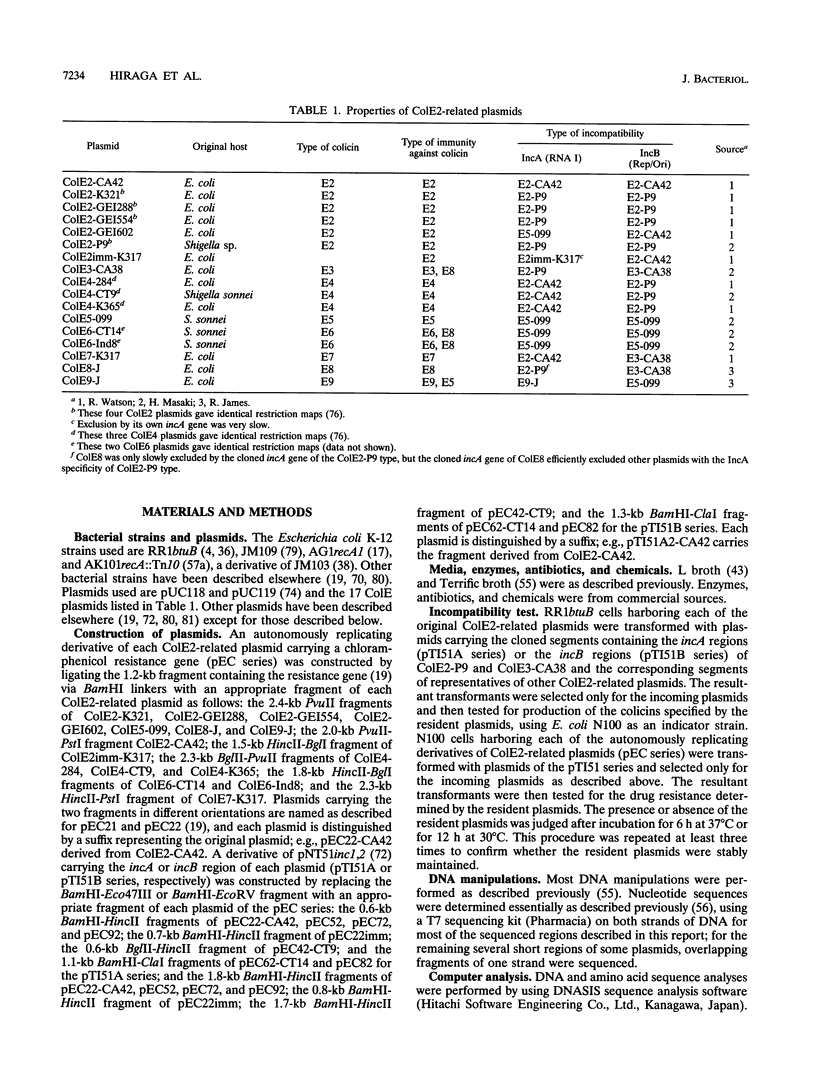
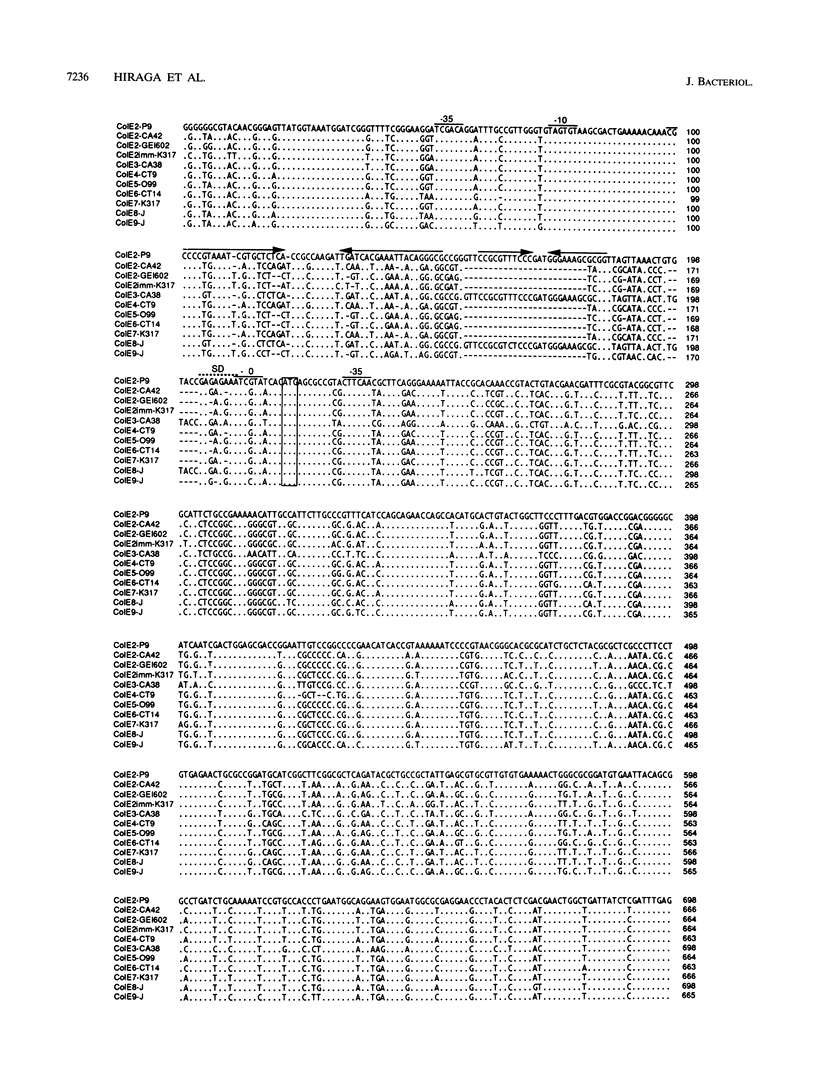
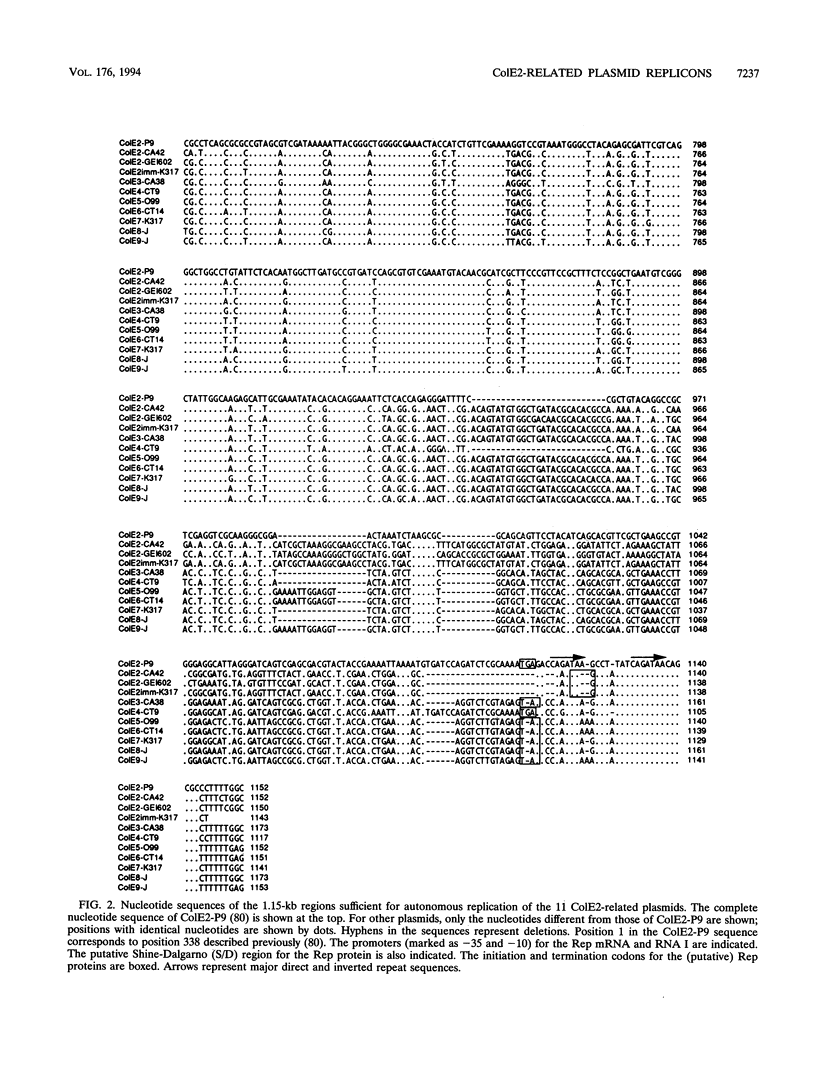
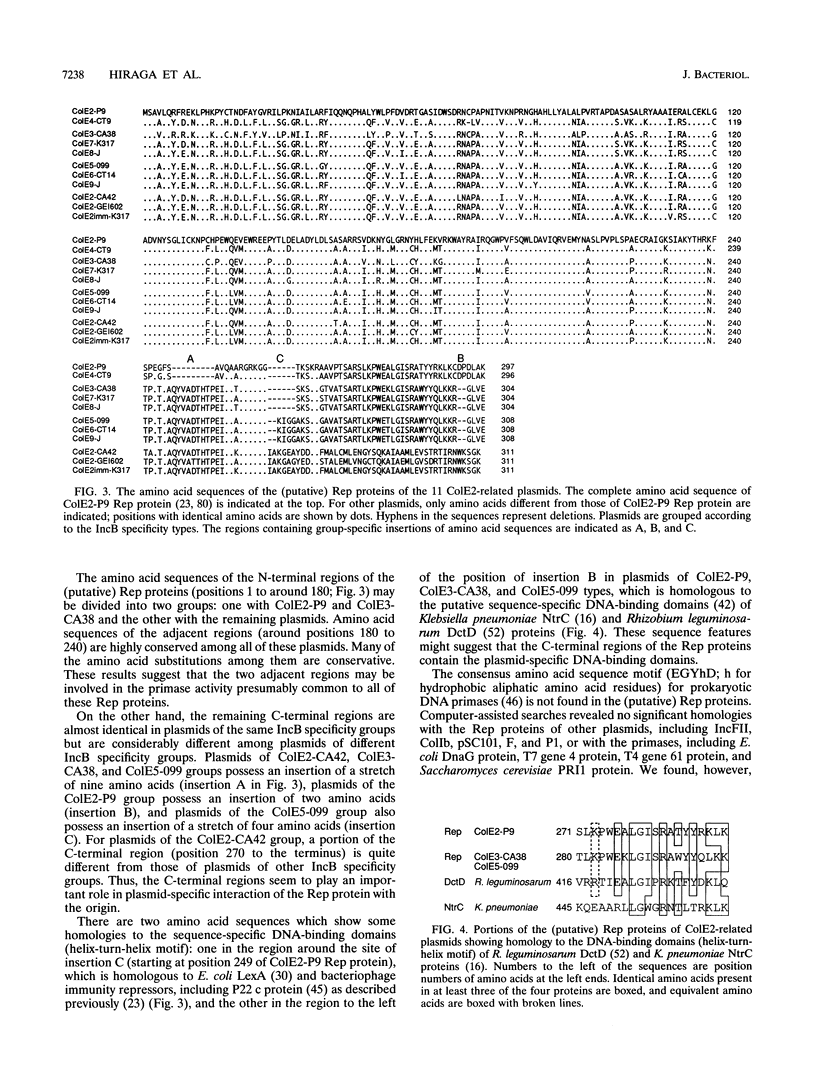
The incA gene product of ColE2-P9 and ColE3-CA38 plasmids is an antisense RNA that regulates the production of the plasmid-coded Rep protein essential for replication. The Rep protein specifically binds to the origin and synthesizes a unique primer RNA at the origin. The IncB incompatibility is due to competition for the Rep protein among the origins of the same binding specificity. We localized the regions sufficient for autonomous replication of 15 ColE plasmids related to ColE2-P9 and ColE3-CA38 (ColE2-related plasmids), analyzed their incompatibility properties, and determined the nucleotide sequences of the replicon regions of 9 representative plasmids. The results suggest that all of these plasmids share common mechanisms for initiation of DNA replication and its control. Five IncA specificity types, 4 IncB specificity types, and 9 of the 20 possible combinations of the IncA and IncB types were found. The specificity of interaction of the Rep proteins and the origins might be determined by insertion or deletion of single nucleotides and substitution of several nucleotides at specific sites in the origins and by apparently corresponding insertion or deletion and substitution of amino acid sequences at specific regions in the C-terminal portions of the Rep proteins. For plasmids of four IncA specificity types, the nine-nucleotide sequences at the loop regions of the stem-loop structures of antisense RNAs are identical, suggesting an evolutionary significance of the sequence. The mosaic structures of the replicon regions with homologous and nonhomologous segments suggest that some of them were generated by exchanging functional parts through homologous recombination.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano K., Kato A., Moriwaki H., Hama C., Shiba K., Mizobuchi K. Positive and negative regulations of plasmid CoLIb-P9 repZ gene expression at the translational level. J Biol Chem. 1991 Feb 25;266(6):3774–3781. [PubMed] [Google Scholar]
- Asano K., Moriwaki H., Mizobuchi K. An induced mRNA secondary structure enhances repZ translation in plasmid ColIb-P9. J Biol Chem. 1991 Dec 25;266(36):24549–24556. [PubMed] [Google Scholar]
- Blakely G., Colloms S., May G., Burke M., Sherratt D. Escherichia coli XerC recombinase is required for chromosomal segregation at cell division. New Biol. 1991 Aug;3(8):789–798. [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Case C. C., Roels S. M., Jensen P. D., Lee J., Kleckner N., Simons R. W. The unusual stability of the IS10 anti-sense RNA is critical for its function and is determined by the structure of its stem-domain. EMBO J. 1989 Dec 20;8(13):4297–4305. doi: 10.1002/j.1460-2075.1989.tb08616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chak K. F., James R. Characterization of the ColE9-J plasmid and analysis of its genetic organization. J Gen Microbiol. 1986 Jan;132(1):61–70. doi: 10.1099/00221287-132-1-61. [DOI] [PubMed] [Google Scholar]
- Chak K. F., James R. Localization and characterization of a gene on the ColE3-CA38 plasmid that confers immunity to colicin E8. J Gen Microbiol. 1984 Mar;130(3):701–710. doi: 10.1099/00221287-130-3-701. [DOI] [PubMed] [Google Scholar]
- Chan P. T., Ohmori H., Tomizawa J., Lebowitz J. Nucleotide sequence and gene organization of ColE1 DNA. J Biol Chem. 1985 Jul 25;260(15):8925–8935. [PubMed] [Google Scholar]
- Colloms S. D., Sykora P., Szatmari G., Sherratt D. J. Recombination at ColE1 cer requires the Escherichia coli xerC gene product, a member of the lambda integrase family of site-specific recombinases. J Bacteriol. 1990 Dec;172(12):6973–6980. doi: 10.1128/jb.172.12.6973-6980.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. C., Hawkins F. K., James R. Incompatibility between E colicin plasmids. J Gen Microbiol. 1986 Jul;132(7):1859–1862. doi: 10.1099/00221287-132-7-1859. [DOI] [PubMed] [Google Scholar]
- Cooper P. C., James R. Two new E colicins, E8 and E9, produced by a strain of Escherichia coli. J Gen Microbiol. 1984 Jan;130(1):209–215. doi: 10.1099/00221287-130-1-209. [DOI] [PubMed] [Google Scholar]
- Couturier M., Bex F., Bergquist P. L., Maas W. K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988 Sep;52(3):375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M. D., James R., Coddington A. An evolutionary relationship between the ColE5-099 and the ColE9-J plasmids revealed by nucleotide sequencing. J Gen Microbiol. 1989 Oct;135(10):2783–2788. doi: 10.1099/00221287-135-10-2783. [DOI] [PubMed] [Google Scholar]
- Dempsey W. B. Transcript analysis of the plasmid R100 traJ and finP genes. Mol Gen Genet. 1987 Oct;209(3):533–544. doi: 10.1007/BF00331160. [DOI] [PubMed] [Google Scholar]
- Drummond M., Whitty P., Wootton J. Sequence and domain relationships of ntrC and nifA from Klebsiella pneumoniae: homologies to other regulatory proteins. EMBO J. 1986 Feb;5(2):441–447. doi: 10.1002/j.1460-2075.1986.tb04230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hjalt T., Wagner E. G. The effect of loop size in antisense and target RNAs on the efficiency of antisense RNA control. Nucleic Acids Res. 1992 Dec 25;20(24):6723–6732. doi: 10.1093/nar/20.24.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T., Itoh T. Replication of ColE2 and ColE3 plasmids: the regions sufficient for autonomous replication. Mol Gen Genet. 1988 May;212(2):225–231. doi: 10.1007/BF00334689. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Incompatibility exhibited by colicin plasmids E1, E2, and E3 in Escherichia coli. J Bacteriol. 1974 Aug;119(2):478–483. doi: 10.1128/jb.119.2.478-483.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Horii T. Replication of ColE2 and ColE3 plasmids: in vitro replication dependent on plasmid-coded proteins. Mol Gen Genet. 1989 Oct;219(1-2):249–255. doi: 10.1007/BF00261184. [DOI] [PubMed] [Google Scholar]
- Kido M., Yasueda H., Itoh T. Identification of a plasmid-coded protein required for initiation of ColE2 DNA replication. Nucleic Acids Res. 1991 Jun 11;19(11):2875–2880. doi: 10.1093/nar/19.11.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Meyer R. J. Copy-number of broad host-range plasmid R1162 is regulated by a small RNA. Nucleic Acids Res. 1986 Oct 24;14(20):8027–8046. doi: 10.1093/nar/14.20.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Korch C., Hagblom P., Ohman H., Göransson M., Normark S. Cryptic plasmid of Neisseria gonorrhoeae: complete nucleotide sequence and genetic organization. J Bacteriol. 1985 Aug;163(2):430–438. doi: 10.1128/jb.163.2.430-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinke L., Wulff D. L. OOP RNA, produced from multicopy plasmids, inhibits lambda cII gene expression through an RNase III-dependent mechanism. Genes Dev. 1987 Nov;1(9):1005–1013. doi: 10.1101/gad.1.9.1005. [DOI] [PubMed] [Google Scholar]
- Kumar C. C., Novick R. P. Plasmid pT181 replication is regulated by two countertranscripts. Proc Natl Acad Sci U S A. 1985 Feb;82(3):638–642. doi: 10.1073/pnas.82.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labidi A., Mardis E., Roe B. A., Wallace R. J., Jr Cloning and DNA sequence of the Mycobacterium fortuitum var fortuitum plasmid pAL5000. Plasmid. 1992 Mar;27(2):130–140. doi: 10.1016/0147-619x(92)90013-z. [DOI] [PubMed] [Google Scholar]
- Lamerichs R. M., Padilla A., Boelens R., Kaptein R., Ottleben G., Rüterjans H., Granger-Schnarr M., Oertel P., Schnarr M. The amino-terminal domain of LexA repressor is alpha-helical but differs from canonical helix-turn-helix proteins: a two-dimensional 1H NMR study. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6863–6867. doi: 10.1073/pnas.86.18.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P. C., Condie J. A. Nucleotide sequences from the colicin E5, E6 and E9 operons: presence of a degenerate transposon-like structure in the ColE9-J plasmid. Mol Gen Genet. 1989 Jun;217(2-3):269–277. doi: 10.1007/BF02464892. [DOI] [PubMed] [Google Scholar]
- Lopez J., Crespo P., Rodriguez J. C., Andres I., Ortiz J. M. Analysis of IncF plasmids evolution: nucleotide sequence of an IncFIII replication region. Gene. 1989 May 15;78(1):183–187. doi: 10.1016/0378-1119(89)90327-2. [DOI] [PubMed] [Google Scholar]
- Males B. M., Stocker B. A. Colicins E4, E5, E6 and A and properties of btuB+ colicinogenic transconjugants. J Gen Microbiol. 1982 Jan;128(1):95–106. doi: 10.1099/00221287-128-1-95. [DOI] [PubMed] [Google Scholar]
- Mark G., Lawrence P., James R. Characterisation of the ColE8 plasmid, a new member of the group E colicin plasmids. Gene. 1984 Jul-Aug;29(1-2):145–155. doi: 10.1016/0378-1119(84)90175-6. [DOI] [PubMed] [Google Scholar]
- Masaki H., Ohta T. Colicin E3 and its immunity genes. J Mol Biol. 1985 Mar 20;182(2):217–227. doi: 10.1016/0022-2836(85)90340-7. [DOI] [PubMed] [Google Scholar]
- McCarthy J. E., Gualerzi C. Translational control of prokaryotic gene expression. Trends Genet. 1990 Mar;6(3):78–85. doi: 10.1016/0168-9525(90)90098-q. [DOI] [PubMed] [Google Scholar]
- Mock M., Pugsley A. P. The BtuB group col plasmids and homology between the colicins they encode. J Bacteriol. 1982 Jun;150(3):1069–1076. doi: 10.1128/jb.150.3.1069-1076.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. A., Robertson C. A. Heteroduplex substrates for bacteriophage lambda site-specific recombination: cleavage and strand transfer products. EMBO J. 1989 Nov;8(11):3523–3533. doi: 10.1002/j.1460-2075.1989.tb08518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoletti S., Bird P., Praszkier J., Pittard J. Analysis of the incompatibility determinants of I-complex plasmids. J Bacteriol. 1988 Mar;170(3):1311–1318. doi: 10.1128/jb.170.3.1311-1318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North A. K., Klose K. E., Stedman K. M., Kustu S. Prokaryotic enhancer-binding proteins reflect eukaryote-like modularity: the puzzle of nitrogen regulatory protein C. J Bacteriol. 1993 Jul;175(14):4267–4273. doi: 10.1128/jb.175.14.4267-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. Analysis of dnaB function of Escherichia coli K12 and the dnaB-like function of P1 prophage. J Mol Biol. 1975 May 25;94(3):327–340. doi: 10.1016/0022-2836(75)90206-5. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Tomizawa J. Nucleotide sequence of the region required for maintenance of colicin E1 plasmid. Mol Gen Genet. 1979 Oct 3;176(2):161–170. doi: 10.1007/BF00273210. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Pansegrau W., Lanka E. A common sequence motif among prokaryotic DNA primases. Nucleic Acids Res. 1992 Sep 25;20(18):4931–4931. doi: 10.1093/nar/20.18.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C., Wagner E. G., Nordström K. Control of replication of plasmid R1: structures and sequences of the antisense RNA, CopA, required for its binding to the target RNA, CopT. EMBO J. 1990 Nov;9(11):3767–3775. doi: 10.1002/j.1460-2075.1990.tb07590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praszkier J., Wei T., Siemering K., Pittard J. Comparative analysis of the replication regions of IncB, IncK, and IncZ plasmids. J Bacteriol. 1991 Apr;173(7):2393–2397. doi: 10.1128/jb.173.7.2393-2397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan S. J., Novick R. Comparative analysis of five related Staphylococcal plasmids. Plasmid. 1988 May;19(3):203–221. doi: 10.1016/0147-619x(88)90039-x. [DOI] [PubMed] [Google Scholar]
- Ranes M. G., Rauzier J., Lagranderie M., Gheorghiu M., Gicquel B. Functional analysis of pAL5000, a plasmid from Mycobacterium fortuitum: construction of a "mini" mycobacterium-Escherichia coli shuttle vector. J Bacteriol. 1990 May;172(5):2793–2797. doi: 10.1128/jb.172.5.2793-2797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauzier J., Moniz-Pereira J., Gicquel-Sanzey B. Complete nucleotide sequence of pAL5000, a plasmid from Mycobacterium fortuitum. Gene. 1988 Nov 30;71(2):315–321. doi: 10.1016/0378-1119(88)90048-0. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Astwood P. M., Nixon B. T., Ausubel F. M. Deduced products of C4-dicarboxylate transport regulatory genes of Rhizobium leguminosarum are homologous to nitrogen regulatory gene products. Nucleic Acids Res. 1987 Oct 12;15(19):7921–7934. doi: 10.1093/nar/15.19.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J., Ryder T., Inokuchi H., Ohtsubo H., Ohtsubo E. Genes and sites involved in replication and incompatibility of an R100 plasmid derivative based on nucleotide sequence analysis. Mol Gen Genet. 1980;179(3):527–537. doi: 10.1007/BF00271742. [DOI] [PubMed] [Google Scholar]
- Saadi S., Maas W. K., Hill D. F., Bergquist P. L. Nucleotide sequence analysis of RepFIC, a basic replicon present in IncFI plasmids P307 and F, and its relation to the RepA replicon of IncFII plasmids. J Bacteriol. 1987 May;169(5):1836–1846. doi: 10.1128/jb.169.5.1836-1846.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer G., Som T., Itoh T., Tomizawa J. The origin of replication of plasmid p15A and comparative studies on the nucleotide sequences around the origin of related plasmids. Cell. 1983 Jan;32(1):119–129. doi: 10.1016/0092-8674(83)90502-0. [DOI] [PubMed] [Google Scholar]
- Siemering K. R., Praszkier J., Pittard A. J. Interaction between the antisense and target RNAs involved in the regulation of IncB plasmid replication. J Bacteriol. 1993 May;175(10):2895–2906. doi: 10.1128/jb.175.10.2895-2906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som T., Tomizawa J. Origin of replication of Escherichia coli plasmid RSF 1030. Mol Gen Genet. 1982;187(3):375–383. doi: 10.1007/BF00332615. [DOI] [PubMed] [Google Scholar]
- Sprengart M. L., Fatscher H. P., Fuchs E. The initiation of translation in E. coli: apparent base pairing between the 16srRNA and downstream sequences of the mRNA. Nucleic Acids Res. 1990 Apr 11;18(7):1719–1723. doi: 10.1093/nar/18.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuitje A. R., Spelt C. E., Veltkamp E., Nijkamp H. J. Identification of mutations affecting replication control of plasmid Clo DF13. Nature. 1981 Mar 19;290(5803):264–267. doi: 10.1038/290264a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Itoh T. Control of ColE2 DNA replication: in vitro binding of the antisense RNA to the Rep mRNA. Nucleic Acids Res. 1993 Dec 25;21(25):5972–5977. doi: 10.1093/nar/21.25.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. K. Derivatives of ColE1 cer show altered topological specificity in site-specific recombination. EMBO J. 1989 Jan;8(1):309–315. doi: 10.1002/j.1460-2075.1989.tb03378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. K., Sherratt D. J. Resolution of ColE1 dimers requires a DNA sequence implicated in the three-dimensional organization of the cer site. EMBO J. 1988 Mar;7(3):851–858. doi: 10.1002/j.1460-2075.1988.tb02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D., Yaish S., Archer J., Sherratt D. Multimer resolution systems of ColE1 and ColK: localisation of the crossover site. Mol Gen Genet. 1985;201(2):334–338. doi: 10.1007/BF00425680. [DOI] [PubMed] [Google Scholar]
- Tacon W., Sherratt D. ColE plasmid replication in DNA polymerase I-deficient strains of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):331–335. doi: 10.1007/BF00582885. [DOI] [PubMed] [Google Scholar]
- Tajima Y., Horii T., Itoh T. Replication of ColE2 and ColE3 plasmids: two ColE2 incompatibility functions. Mol Gen Genet. 1988 Nov;214(3):451–455. doi: 10.1007/BF00330479. [DOI] [PubMed] [Google Scholar]
- Takechi S., Yasueda H., Itoh T. Control of ColE2 plasmid replication: regulation of Rep expression by a plasmid-coded antisense RNA. Mol Gen Genet. 1994 Jul 8;244(1):49–56. doi: 10.1007/BF00280186. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. Control of ColE1 plasmid replication: initial interaction of RNA I and the primer transcript is reversible. Cell. 1985 Mar;40(3):527–535. doi: 10.1016/0092-8674(85)90201-6. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T. Plasmid ColE1 incompatibility determined by interaction of RNA I with primer transcript. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6096–6100. doi: 10.1073/pnas.78.10.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. H., Sugimoto N., Freier S. M. RNA structure prediction. Annu Rev Biophys Biophys Chem. 1988;17:167–192. doi: 10.1146/annurev.bb.17.060188.001123. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Vernet T., Visentin L. P. Relationships of the Col plasmids E2, E3, E4, E5, E6, and E7: restriction mapping and colicin gene fusions. Plasmid. 1985 May;13(3):205–210. doi: 10.1016/0147-619x(85)90044-7. [DOI] [PubMed] [Google Scholar]
- Watson R., Rowsome W., Tsao J., Visentin L. P. Identification and characterization of Col plasmids from classical colicin E-producing strains. J Bacteriol. 1981 Aug;147(2):569–577. doi: 10.1128/jb.147.2.569-577.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Palchaudhuri S. Incompatibility repressor in a RepA-like replicon of the IncFI plasmid ColV2-K94. J Bacteriol. 1986 Jun;166(3):1106–1112. doi: 10.1128/jb.166.3.1106-1112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yasueda H., Horii T., Itoh T. Structural and functional organization of ColE2 and ColE3 replicons. Mol Gen Genet. 1989 Jan;215(2):209–216. doi: 10.1007/BF00339719. [DOI] [PubMed] [Google Scholar]
- Yasueda H., Takechi S., Sugiyama T., Itoh T. Control of ColE2 plasmid replication: negative regulation of the expression of the plasmid-specified initiator protein, Rep, at a posttranscriptional step. Mol Gen Genet. 1994 Jul 8;244(1):41–48. doi: 10.1007/BF00280185. [DOI] [PubMed] [Google Scholar]
- Zverev V. V., Khmel I. A. The nucleotide sequences of the replication origins of plasmids ColA and ColD. Plasmid. 1985 Nov;14(3):192–199. doi: 10.1016/0147-619x(85)90002-2. [DOI] [PubMed] [Google Scholar]


