Abstract
Modulatory actions of monoamines were investigated on spinal commissural interneurons which coordinate left-right hindlimb muscle activity through direct projections to the contralateral motor nuclei. Commissural interneurons located in Rexed lamina VIM, with identified projections to the contralateral gastrocnemius-soleus motor nuclei, were investigated in deeply anaesthetized cats. Most interneurons had dominant input from either the reticular formation or from group II muscle afferents; a small proportion of neurons had input from both. Actions of ionophoretically applied serotonin and noradrenaline were examined on extracellularly recorded spikes evoked monosynaptically by group II muscle afferents or reticulospinal tract fibres. Activation by reticulospinal fibres was facilitated by both serotonin and noradrenaline. Activation by group II afferents was also facilitated by serotonin but was strongly depressed by noradrenaline. To investigate the possible morphological substrates of this differential modulation, seven representative commissural interneurons were labelled intracellularly with tetramethylrhodamine-dextran and neurobiotin. Contacts from noradrenergic and serotoninergic fibres were revealed by immunohistochemistry and analysed with confocal microscopy. There were no major differences in the numbers and distributions of contacts among the interneurons studied. The findings suggest that differences in modulatory actions of monoamines, and subsequent changes in the recruitment of subpopulations of commissural interneurons in various behavioural situations, depend on intrinsic interneuron properties rather than on the patterns of innervation by monoaminergic fibres. The different actions of noradrenaline on different populations of interneurons might permit reconfiguration of the actions of the commissural neurons according to behavioural context.
Keywords: cat, confocal microscopy, group II afferents, reticular formation, spinal cord
Introduction
Spinal commissural interneurons that project to α-motoneurons on the contralateral side of the spinal cord are of particular importance for the coordination of activity of muscles on the right and left side of the body. It has long been known that muscles may operate within several motor synergies, being activated on one side and inhibited on the other in some movements, but coactivated or coinhibited in other movements (Sherrington, 1906). More recent studies have shown that the selection of a motor synergy is both state and phase dependent (Grillner & Rossignol, 1978; Rossignol & Gauthier, 1980; Gauthier & Rossignol, 1981; Rossignol et al., 1981; Pearson & Rossignol, 1991) and that it involves activation of the most appropriate neuronal networks including those of commissural interneurons (Butt & Kiehn, 2003).
The selection of commissural interneurons mediating crossed inhibition or crossed excitation of hindlimb motoneurons from group II muscle spindle afferents has been shown to depend on modulatory actions of serotonin. Short latency inhibition of contralateral extensor motoneurons is present in the intact spinal cord (Aggelopoulos & Edgley, 1995). Following transection of the spinal cord this inhibition disappears and the reflex pattern reverts to excitation (Arya et al., 1991) but the inhibition reappears following administration of the 5-HTla and 5-HT7 receptor agonist (+/−)-8-hydroxy-2-(di-n-propylamino)-tetralin-hydrobromide (8-OH-DPAT (Aggelopoulos et al., 1996b). This suggests that activation of excitatory and inhibitory group II commissural interneurons is modulated by descending serotoninergic control. Modulatory actions of noradrenaline on these interneurons have not been investigated. As a whole, the population of commissural interneurons (see Cajal, 1953; Scheibel & Scheibel, 1966; Stokke et al., 2002) is highly nonhomogenous. Even within Rexed lamina VIII, there are subpopulations of interneurons with predominant input from group Ia and Ib muscle afferents (Harrison et al., 1986), from group II muscle and skin afferents (Jankowska & Noga, 1990) and from descending reticulo- and vestibulospinal fibres (Bannatyne et al., 2003; Jankowska et al., 2003; Krutki et al., 2003). The interneurons with dominant input from group II muscle afferents or from the reticular formation (RF) appear to be largely separate populations, and these populations may not all be affected by mono-amines in the same way. One aim of this study was therefore to compare the modulatory actions of two monoamines, serotonin (5-HT) and noradrenaline (NA), on commissural interneurons with dominant group II or RF input. In order to relate the comparison to the functional types of neuron, we selected interneurons located within the same part of the spinal cord (in lamina VIII or in the adjacent part of lamina VII), in the same segments of the spinal cord (the L4 segment and adjacent parts of the L3 and L5 segments), and with similar projections (all of them were antidromically activated from the contralateral gastrocnemius-soleus motor nuclei in L7-S1). The second aim was to examine whether any differences in modulatory actions of monoamines are associated with morphological differences in relations between serotoninergic and noradrenergic nerve fibres and interneurons with different input, by using confocal microscopy.
Methods
Preparation
The experiments were performed on 10 deeply anaesthetized cats of both sexes, aged 5–14 months, weighing 2.3–3.2 kg and obtained from a supplier accredited by Göteborg University. All experimental procedures were approved by Göteborg University Ethics Committee and followed NIH and EU guidelines of animal care. Anaesthesia was induced with sodium pentobarbital (40–44 mg/kg, i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; doses of 5 mg/kg administered every 1–2 h, up to 50 mg/kg, i.v.). During recordings, neuromuscular transmission was blocked with pancuronium bromide (Pavulon, Organon, Sweden; ≈ 0.2mg/kg/h i.v.) and the animals were artificially ventilated. Additional doses of α-chloralose were given at the first sign of any increase in the blood pressure or heart rate, continuously monitored, or if the pupils dilated in response to noxious stimulation. Mean blood pressure was kept at 100–130 mmHg and the end-tidal concentration of CO2 at ≈ 4% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (l–2mL/h/kg). The core body temperature was kept at ≈ 37.5 °C by servo-controlled infrared lamps. The experiments were terminated with a lethal dose of pentobarbital resulting in cardiac arrest unless the experiment involved neuronal labelling, in which case the experiments terminated (≈ 2 h after injection of the last neuron) with a near lethal dose of pentobarbital followed by exsanguination and formalin perfusion.
A preliminary dissection exposed the third to seventh lumbar (L3–L7) segments of the spinal cord and a number of peripheral hindlimb nerves which were transected and mounted on stimulating electrodes. Subcutaneous cuff electrodes were used for nerves accessed in the iliac fossa [ipsilateral quadriceps (Q) and sartorius (Sart) nerves and contralateral Q (coQ) nerves], the remaining ipsilateral nerves (the posterior biceps and semitendinosus, PBST, anterior biceps and semi-membranosus, ABSM, anterior tibial and extensor digitorum longus nerves jointly referred to as deep peroneal, DP, and gastrocnemius-soleus GS) were mounted on pairs of silver hook electrodes in a paraffin oil pool created by skin flaps and heated to 37.5 °C.
For stimulation of reticulospinal neurons, the caudal part of the cerebellum was exposed with a craniotomy, and a tungsten electrode (impedance 70–300 Ω) placed either within or just lateral to the ipsilateral medial longitudinal fasciculus (MLF). The electrode was inserted at an angle of 30° (with the tip directed rostrally). The initial target was at Horsley-Clarke co-ordinates P 8–9; L 0.8–1.2 and H –5, but the final position of the electrode was adjusted in each animal on the basis of records of descending volleys evoked by single stimuli from the surface of the lateral funiculus at the Th11–Th13 level, so that the electrodes were left at sites from which distinct descending volleys at a latency of 2.0–2.2 ms were evoked at stimulus thresholds of 20–50 μA. At the end of the experiments these sites were marked by passing 0.4-mA constant current for 10s. The locations of stimulation sites were subsequently verified on 100-μm-thick frontal sections of the brain stem (Fig. 1A), cut in the plane of insertion of the electrodes using either a vibratome or a freezing microtome, and counterstained with Cresyl Violet.
Fig. 1.
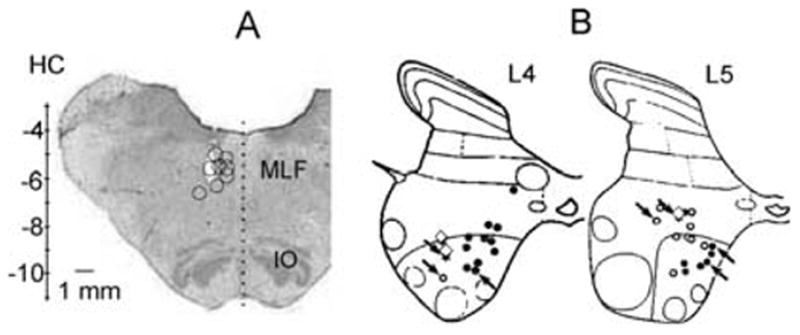
Locations of stimulation sites in the reticular formation and of the sampled commissural interneurons. (A) Transverse section of the medulla in the plane of the insertion of the electrodes at the level of the inferior olive. Open circles indicate stimulation sites in the MLF. Vertical scale shows Horsley-Clarke coordinates. (B) Summary diagrams showing the locations of intracellularly labelled commissural neurons in the L4 and L5 segments of the spinal cord. ○, ●, cells with monosynaptic input from group II afferents and from RF, respectively. The three ◇ represent the locations of three interneurons with short-latency input from both. Arrows indicate the interneurons in which the distribution of 5-HT and NA contacts was analysed in detail. IO, inferior olive.
Stimulation and recording
Peripheral nerves were stimulated with constant voltage stimuli (0.1 ms duration, intensity expressed in multiples of threshold, T, for the most sensitive fibres in the nerve). For activation of group II afferents, stimuli at intensities of 3–5 × T, near maximum for fast conducting group II afferents (Jack, 1978), were used. For activation of fibres of the reticulospinal tract, constant-current stimuli (0.2 ms, 50–200 μA) were applied using a 0.5-mm electrolytically etched tungsten wire, insulated except for its tip as a cathode (Jankowska et al., 2003). Axons of commissural interneurons terminating in the motor nuclei were stimulated (single pulses, 0.2ms, 6–50 μA) using a similar but thinner tungsten electrode which replaced a glass micropipette with which the nuclei were initially located.
In experiments in which the effects of ionophoretically applied monoamines were tested, glass micropipettes (tip diameter ≈ 1.5 μm) filled with 2 M NaCl solution were used for extracellular recording and micropipettes (tip diameter ≈ 2 μm) filled with a solution of the investigated agents (see details below) were used for ionophoresis. In the experiments involving intracellular recording and labelling, glass micropipettes (≈ 1.5 μm tip diameter), filled either with a 2% solution of tetramethylrhodamine-dextran (Molecular Probes, Inc, Eugene, Oregon, USA) in saline (pH 6.5) or with a mixture of equal parts of (5%) tetramethylrhodamine-dextran and (5%) neurobiotin (Vector) were used.
Sampling
Cells were searched for in the L3–L5 segments of the spinal cord, at locations where large distinct focal synaptic field potentials were evoked from group II (but not group I) afferents and/or from the MLF (depths between 2.8 and 3.7 mm from the surface of the spinal cord), depending on the segment (see Edgley & Jankowska, 1987). Cells were selected on the basis of monosynaptic excitation by either group II afferents (of Q, Sart and DP, the main sources of group II input in the L3–L5 segments) or from reticulospinal tract fibres. All of the cells were identified as commissural interneurons by antidromic activation from within the contralateral gastrocnemius-soleus motor nuclei in the caudal part of the L7 segment. As a rule, currents of <50 μA (0.2 ms duration) were used, giving an estimated spread of current not exceeding 0.5mm (Gustafsson & Jankowska, 1976). In order to differentiate between antidromic and synaptic activation of extracellularly recorded cells, only those activated with short latencies (1–1.5ms) and in which antidromic action potentials collided with synaptically evoked action potentials, were accepted. In intracellularly recorded cells antidromic activation was based on the all-or-none appearance of either full or blocked action potentials which were not preceded by EPSPs (e.g. Fig. 6). All of these neurons were mono-synaptically excitated by either group II afferents in the Q or Sart nerves, or following MLF stimuli. Both extracellularly recorded spike potentials and intracellularly recorded EPSPs were adjudged as evoked monosynaptically when they were induced, by maximal stimuli, at latencies not exceeding 3.0ms from group I afferent volleys (but allowance was made for an additional 0.5 ms for the three extracellularly recorded neurons which were very weakly activated) and of up to 1.2ms from the first component of the RF descending volley (see Edgley & Jankowska, 1987; Jankowska et al., 2003). Lack of antidromic activation from the thoracic segments was used to differentiate commissural interneurons from ascending tract neurons with local spinal collaterals. The thoracic stimuli were applied by pairs of silver ball electrodes in contact with the left and right lateral funiculi at an intensity (0.5–1 mA, 0.2 ms) at which other ventral horn neurons were antidromically activated.
Fig. 6.
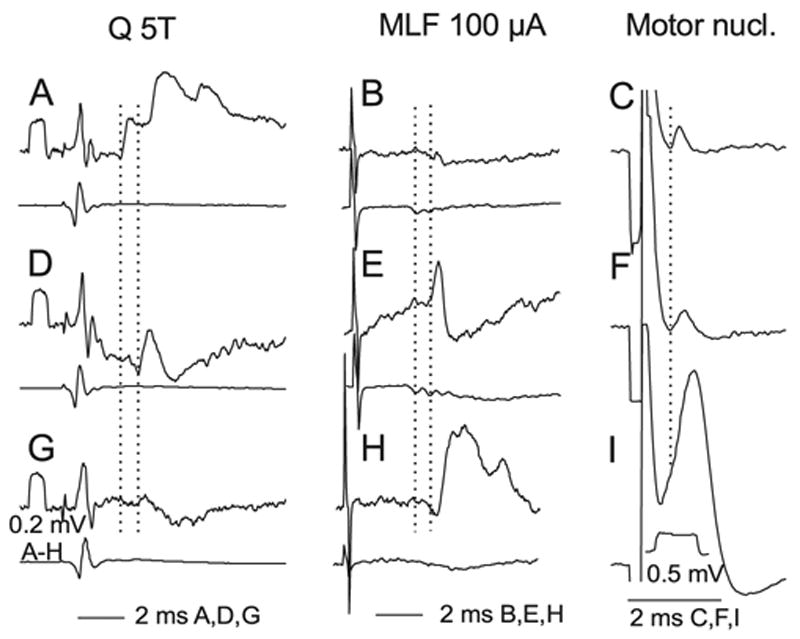
Examples of intracellular records from labelled interneurons. The three rows show intracellular records (upper traces) from three interneurons (nos 2, 6 and 3 from Table 1), together with records of afferent or descending volleys (lower traces). The left column shows effects of stimulation of group II afferents in the Q nerve; (A) monosynaptic and disynaptic EPSPs, (D) an EPSP that is either monosynaptic or disynaptic, and (G) a disynaptic IPSP (possibly preceded by a very small EPSP as in D). The dotted vertical lines indicate the onset of monosynaptic and most probably disynaptic PSPs. The middle column shows the effects of stimulation of reticulospinal tract fibres: evoking a monosynaptic EPSP in H, a monosynaptic EPSP followed by IPSP in E and no clear response in B. Dotted lines indicate the first components of the RF descending volleys and onset latencies of the earliest monosynaptic EPSPs. The right-hand column shows the effects of stimuli applied in GS motor nuclei: small initial segment and soma-dendritic spike in I and blocked spikes in C and F. These impaled neurons ceased generating full actions potentials briefly after penetration. The dotted vertical line indicates onset of the two earliest spikes.
lonophoresis
The two compounds used were 5-hydroxytryptamine (5-HT, serotonin; Sigma) and noradrenaline (NA, Sigma). They were applied locally by ionophoresis from a 0.2M solution in H2O, pH 4.5, using a double-headed micromanipulator with two separate microdrives for the recording and the drug-containing micropipettes (Engberg et al., 1972). This permitted the use of a single recording pipette when searching for and characterizing interneurons; subsequently, while maintaining the recording, the drug-containing pipette could be inserted and moved to a position close to the recording pipette (with the tips of the two pipettes 5–10 μm apart; see Jankowska et al., 2000; Hammar & Jankowska, 2003). During the initial insertion of the ionophoresis pipette a 10-nA retaining current was used to reduce the possibility of leakage though the tip of the drug-containing micropipette. For each tested interneuron, two series of control records were taken before the ionophoresis began: one before the insertion of the drug-containing pipette (control) and the other when the latter had reached its position close to the recording pipette but before ejecting the drug (placement), in order to make sure that the placement of the micropipette did not alter the responses. The drugs were ejected by passing a negative current of 20 nA for a period of up to 3 min whilst simultaneously recording from the neuron through the recording micropipette. During ionophoresis the shape and amplitude of a current pulse applied through the drug-containing pipette were monitored to ensure that its resistance did not increase excessively, which would indicate blockage. Following the termination of the ionophoresis, the drug-containing pipette was withdrawn from the spinal cord and the responses of the neurons were recorded during a recovery period lasting up to 25 min.
Electrophysiological analysis of effects of monoamines
Responses to 20 consecutive stimuli were sampled every 15 s for as long as the ionophoresis continued and thereafter every 2–5 min during the recovery period. Effects of the ionophoresed substances were evaluated by comparing the number of responses to the 20 stimuli and any changes in response latency before, during and after ionophoresis. Peri-stimulus time histograms and cumulative sum plots were created on-line and stored in parallel with the original data records. In order to ensure that the response probability was evaluated with sufficient sensitivity, the stimuli used to evoke responses were adjusted (stimulus intensity and/or numbers of stimuli) until responses were evoked in ≈ 50% or less of stimulus deliveries; both increases and decreases in response probability could thus be identified. In order to restrict the tests to responses evoked monosynaptically, time windows of 1 ms were used (as measured from the earliest response at latencies compatible with a monosynaptic coupling; see above). Drawing on results obtained in previous studies of effects of ionophoresis of 5-HT and NA (Bras et al., 1989; Hammar et al., 2002) separate control experiments passing current through a micropipette filled with an HCl solution at pH 4.5 in order to exclude direct actions of H+ ions were not performed.
Data are expressed as means ± SEM. Stastical significance was calculated using the Wilcoxon signed rank test, using Statview software.
Labelling and perfusion
After penetration and identification, neurons selected for morphological analysis were labelled ionophoretically. Tetramethylrhodamine-dextran was ejected by passing a constant positive current of 5–10 nA through the micropipette for up to 10 min, while continuous recordings verified that the electrode remained intracellular. In the successfully labelled cells, the total product of nA × min ranged between 16 and 50. The tetramethylrhodamine-neurobiotin mixture was ejected by passing 5nA for 6–10 min with the total product of between 18 and 45 nA × min. In both cases a few minutes were allowed for the markers to diffuse before the pipette was withdrawn. A minimum space of 800 mm was allowed between labelled neurons. At the conclusion of experiments, the descending aorta was canulated and animals were perfused, initially with 0.9% NaCl in 0.1 M phosphate buffer (pH 7.4) and subsequently with 2 L of fixative (4% formaldehyde in 0.1 M phosphate buffer, pH 7.4). Blocks of spinal cord (5–6 mm in length) containing the labelled cells were removed and placed in the same fixative at 4°C for 8h.
Processing for confocal microscopy
Transverse vibratome sections (50 μm thick) were cut and collected in strict serial order. Sections from experiments in which tetramethylrhodamine-dextran was injected were incubated in 50% ethanol for 30 min and mounted in Vectashield. Sections in which cells had been labelled with both rhodamine and neurobiotin were incubated in 50% ethanol for 30 min, washed several times in PBS and further incubated with avidin-rhodamine (1 : 1000) for 3 h. Sections were scanned using epifluorescence and those containing labelled cells were processed further. Monoamine-containing terminals were revealed by incubating sections individually in a blocking serum containing 10% normal donkey serum in PBS with 0.3% Triton for 30 min. They were then placed in solutions of primary antibodies (either rat anti-5HT or rabbit antidopamine β-hydroxylase (d.b.h.) at concentrations of 1 : 200 and 1 : 500, respectively (both supplied by Affiniti Research Products, Nottingham, UK) for 48 h at 4 °C. Following several further washes in PBS, the sections were incubated with secondary antibodies, donkey antirabbit (1 : 100) conjugated with Fluorescein isothiocyante (FITC) and donkey antirat (1 : 100) conjugated with Cyanine-5 (Cy-5, both supplied by Jackson ImmunoResearch, Luton, UK). Sections were then remounted in Vectashield and stored at −20 °C.
Confocal microscopy analysis
Although 30 neurons were labelled, seven were selected for detailed confocal analysis on the basis of the quality of labelling (five labelled with tetramethylrhodamine-dextran and two with the mixture of tetramethylrhodamine-dextran and neurobiotin). Cells were scanned with a three-channel confocal laser scanning microscope (Biorad MRC 1024, Hemmel Hempstead, UK) before reconstruction using NeuroLucida for Confocal software (MicroBrightField, Colchester, VT, USA), which permits three-dimensional reconstruction of cells and also allows mapping of contacts. Putative contacts were examined closely and only defined as appositions if the two labelled profiles were immediately adjacent with no intervening black pixels. The distribution of terminals was studied by using Sholl analysis to determine the numbers of contacts per 100 μm of dendritic length within series of concentric spheres originating at the centre of the soma. Contact densities were calculated using data generated by NeuroLucida and expressed as numbers of contacts per 100 μm2 of neuronal surface area.
Results
Effects of monoamines on lamina VIII commissural interneurons
Effects of ionophoretically applied monoamines were investigated on responses evoked by group II afferents (n = 17) or by reticulospinal tract fibres (n = 19). These were as a rule evoked in different commissural interneurons, except for one interneuron which was tested for effects of 5-HT which was activated at latencies compatible with monosynaptic coupling by both group II and RF fibres. The data are therefore presented as for two nonoverlapping neuronal populations. However, weak input from one of these sources might have remained undetected, because we used extracellular recording for the purpose of ionophoresis. Intracellular records from cells that were subsequently labelled intracellularly revealed a larger (albeit still relatively small) proportion (17.5%) of commissural interneurons in which both group II afferents and RF-evoked short latency responses. These included four interneurons with apparently monosynaptic EPSPs from both group II afferents and RF, and three interneurons with monosynaptic EPSPs from RF and apparently disynaptic EPSPs from group II afferents.
Effects of 5-HT
Activation of all of the investigated commissural interneurons was facilitated following ionophoretic application of 5-HT. Figure 2 illustrates the effect for two commissural interneurons, one of which was monosynaptically activated by RF stimulation (A) and the other was activated by group II afferents (B). As shown by the histograms (Fig. 2C and D), this was a potent and robust effect; the numbers of spikes evoked by the sequence of 20 stimuli were nearly doubled in both cases. Some differences were, however, noted between commissural interneurons with the two kinds of input. The effects of 5-HT seemed to start earlier when tested on responses evoked by reticulospinal tract neurons (as exemplified in Fig. 2C and summarized in Fig. 4C) than on those evoked by group II afferents (Figs 2D and 4D) and the duration of the facilitation seemed longer lasting in neurons activated by group II afferents.
Fig. 2.
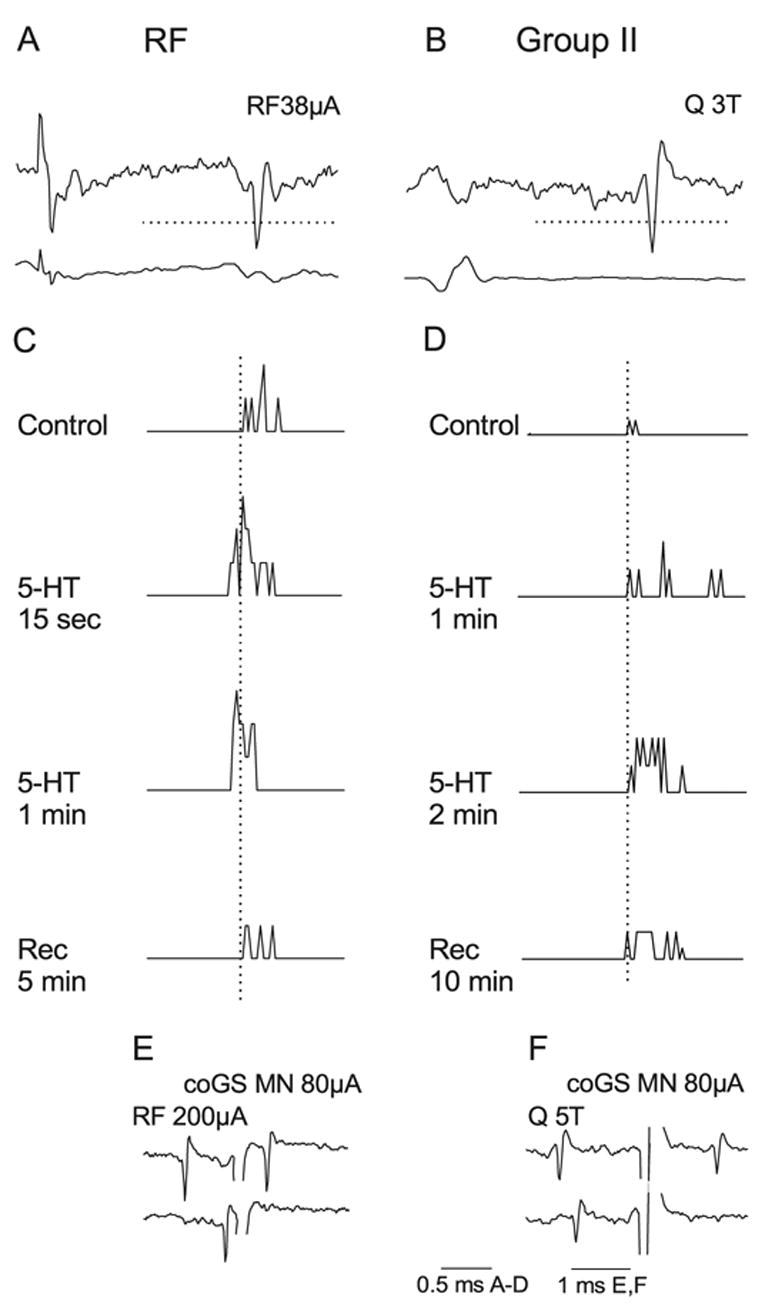
Similar facilitatory effects of 5-HT on commissural interneurons activated from RF and by group II afferents. (A and C) Top pair of records are single sweep records from an interneuron monosynaptically activated by RF fibres and from the cord dorsum. The traces below are peri-stimulus time histograms (PSTH) of responses to 20 stimuli. They were complied from stimuli delivered before (control), during (15s, 1 and 2min) and after ionophoresis of 5-HT (Rec., 5 min after withdrawal of the ionophoretic pipette). (B and D) The same format as in A and C but for an interneuron activated by stimulation of group II afferents in the Q nerve. Dotted vertical lines in C and D indicate the minimal latency in the control records. The dotted horizontal lines in A and B indicate the discrimination levels; only spikes crossing these lines were used for construction of PSTHs. (E and F) Collision tests for the neurons in A and B, respectively. The records show that antidromic spikes from the contralateral GS motor nucleus collided with synaptically evoked responses at intervals shorter than twice the conduction time from this nucleus. Shock artefacts truncated. Time calibrations as indicated.
Fig. 4.
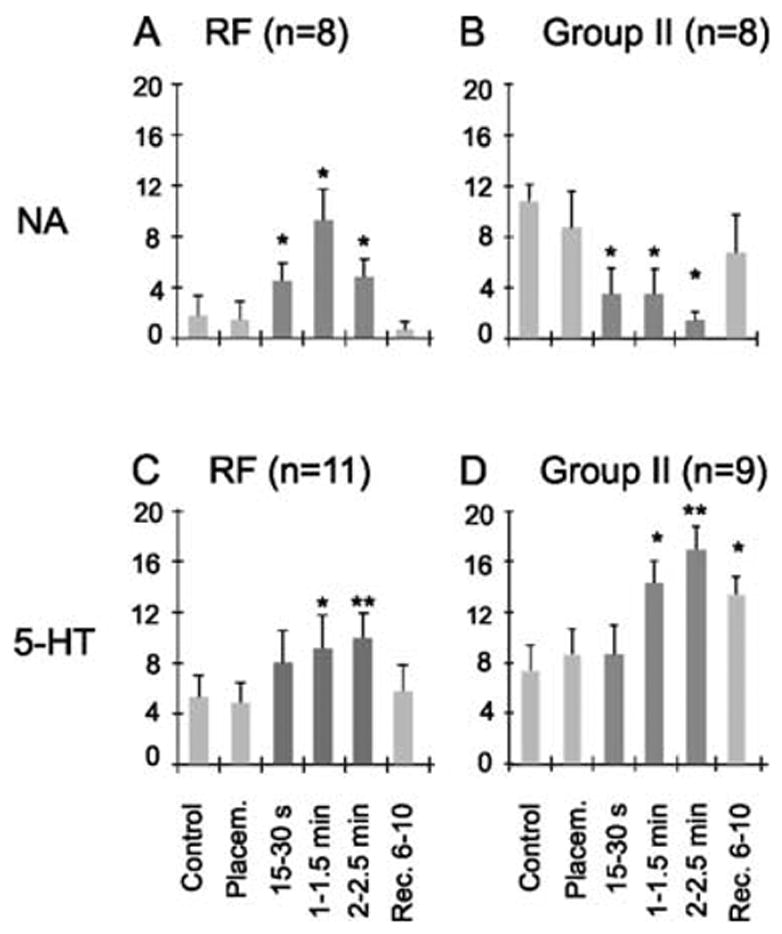
Effects of monoamines on two subpopulations of commissural interneurons. The plots show changes in the number of spikes evoked during a series of 20 consecutive stimuli following ionophoresis of noradrenaline (NA; upper panels) or serotonin (5-HT; lower panels) on lamina VIII commissural interneurons activated by either the reticulospinal tract fibres (left panels) or by group II afferents (right panels). The ordinates show the mean number of responses (± SEM) before, during (dark grey) or after ionophoresis at the times indicated. Control and placement (Placem.), responses evoked before and after placement of the drug-containing microelectrode, respectively. Other data are for the time periods indicated below the bars during ionophoresis and after up to 10 min of recovery (Rec.). *P = 0.05–0.01, *P < 0.01 vs. control levels.
In six of 11 RF-activated neurons clear facilitatory effects, which were statistically significant (P < 0.05), were seen only 15s after ionophoresis began. After 1 min of ionophoresis the mean number of responses had significantly increased for all neurons and the mean number of responses (to 20 consecutive stimuli) had increased from 5.2 ± 1.5 to 9.1 ± 2.6 (P < 0.01).
In contrast, after 15 s of ionophoresis only two of the nine neurons activated from group II afferents showed facilitated activation and statistically significant differences for all these interneurons were not found until 1–1.5 min of ionophoresis. At this time the mean number of responses increased from 7.3 ± 2.0 to 14.2 ± 1.7 (P < 0.05). The apparently slower-developing facilitation of activation of these neurons appeared to be associated with a slower recovery than of RF-activated interneurons. The number of responses of group II-activated interneurons after 1–1.5 min of ionophoresis remained similar for 6–10 min after the ionophoresis pipette was removed, and slowly returned to preionophoresis levels after 20–30 min. In contrast, the responses of RF-activated neurons had practically all returned to control levels 10 min after terminating the ionophoresis.
The 5-HT mediated facilitation, expressed as an increase in the number of responses with which they responded to the stimuli, was sometimes associated with a decrease in response latency, as in Fig. 2C. However, this was seen in only four neurons of each sub-population and varied substantially for individual neurons during ionophoresis.
Effects of NA
Unlike the effects of 5-HT, which were facilitatory on all neurons, NA facilitated only activation from RF whereas it depressed activation from group II afferents. The depression of responses evoked from group II afferents by NA was very effective. As shown in Fig. 4B the onset of the depression was prompt, with a significant decrease in the number of responses (from a mean of 10.5 ± 2.4 to a mean of 3.5 ± 2.3, P < 0.05) seen 15s after the onset of ionophoresis in all of the eight investigated neurons. In addition, any responses evoked during ionophoresis generally appeared at longer latencies, as exemplified in Fig. 3D.
Fig. 3.
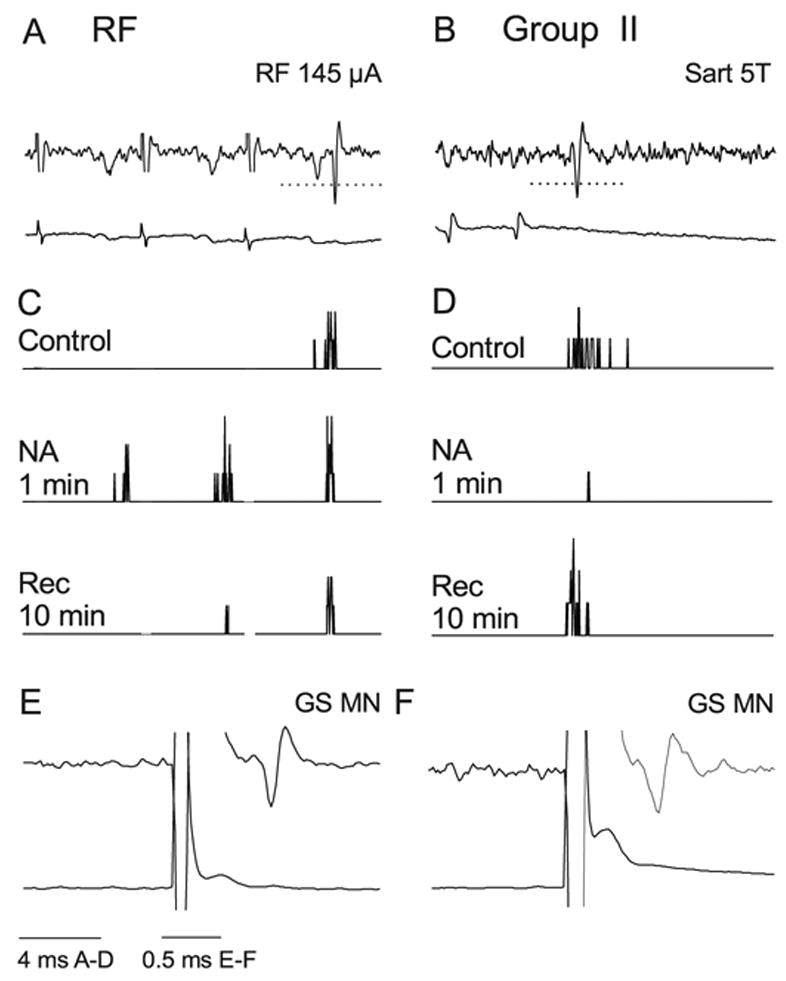
Facilitation of responses to RF stimulation and depression of responses to group II activation by NA. (A and B) Extracellular records of monosynaptic responses to stimulation in the RF and of group II afferents in the Sart nerve in two different commissural interneurons and records from cord dorsum. Shock artefacts have been truncated. (B and D) PSTHs of responses evoked by 20 consecutive stimuli recorded before (control), during and after ionophoresis of NA. Note that in B only the third stimulus evoked spikes during the control period, whereas the first and second stimulus also evoked spikes during ionophoresis. The facilitated responses which appeared after the first stimulus were used for the pooled data of Fig. 4. The dotted horizontal lines in A and B indicate the discrimination level; only spikes crossing these lines were used for constructing the histograms. (E and F) Spike potentials evoked from GS motor nucleus; latencies < 1 ms show that they were evoked antidromically.
Facilitation of responses evoked from RF was similarly effective. It was potent enough to induce responses to stimuli that were ineffective in the control period. For example, we routinely used trains of two or three RF stimuli where only the last stimulus evoked responses in the control period, whereas during ionophoresis the first stimulus also evoked spikes in some trials (in five of the eight interneurons tested; see Fig. 3A and C). The facilitation appeared almost immediately following the start of drug application and the increase in the mean number of responses (from 1.6 ± 1.6 to 4.5 ± 1.3) was statistically significant (P < 0.05) after 30 s, as shown in Fig. 4A. Interestingly, the facilitation decreased somewhat during ionophoresis after drug application for > 1.5 min (in four of the eight neurons tested). Why this occurred is not known, but an increase in the impedance of the drug-containing pipette and a decrease in the amount of NA ejected could have contributed.
Effects on responses from contralateral group II afferents
Stimulation of contralateral group II afferents from the quadriceps (coQ) nerve excited a proportion (11/32) of the commissural interneurons, including some with monosynaptic RF input (n = 7) and some that were monosynaptically activated by group II afferents (n = 4). The latencies of responses evoked from coQ ranged from 4.4 to 7.8 ms in RF-activated neurons, and from 3.5 to 5.8ms in group II-activated interneurons, thus most often being compatible with a polysynaptic linkage, but for those of 3.5–4.5 ms with a trisynaptic linkage (see fig. 12 in Jankowska et al., 2002b).
Modulation of responses evoked from contralateral group II afferents is of interest for the interpretation of the results presented above, because presynaptic actions of monoamines on terminals of group II afferents, which were on the other side of the spinal cord, could be excluded as a contributing factor.
Effects of 5-HT were investigated on four of these neurons in parallel with effects of 5-HT on monosynaptic responses, two from RF and two from ipsilateral group II afferents. All of them were found to be facilitatory. Only one of these neurons responded reliably to stimulation of coQ during the control and placement periods prior to ionophoresis. In the three remaining neurons, one of which is illustrated in Fig. 5A–C, the responses appeared only during ionophoresis. As shown in Fig. 5C, the facilitation and the recovery of responses evoked from coQ [grey peri-stimulus time histograms (PSTHs] paralleled those of responses from RF (black PSTHs) but was even more conspicuous in view of the lower control values.
Fig. 5.
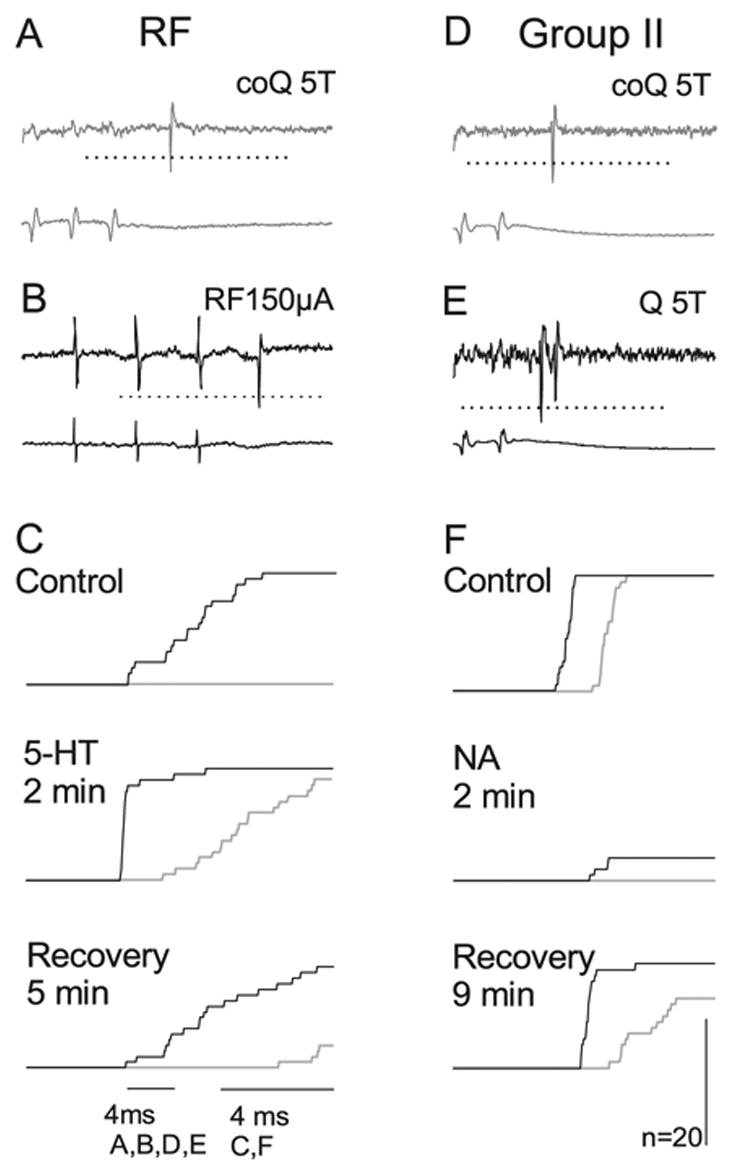
Similar effects of 5-HT and NA on responses evoked by coQ and from ipsilateral Q afferents or RF. Responses of two commissural interneurons are shown, one (A–C) activated by RF and the other (D–F) monosynaptically activated by ipsilateral group II afferents. A and D show responses evoked by stimulation of coQ at 5 × T during ionophoresis (two stimuli in A, two in D). B and E show responses of the same neurons following stimulation of the ipsilateral reticulospinal tract fibres and ipsilateral Q at 5 × T, respectively. (C) Superimposed cumulative sums of responses, from RF (black) and from coQ (grey) show the number of responses evoked before (control) during (2 min of ionophoresis) and after ionophoresis of 5-HT. Cumulative sums were constructed from the bin values of PSTHs like those in Figs 2 and 3. Note that stimulation of coQ did not evoke responses before the ionophoresis began, and that the number of responses was greatly reduced and the latency increased 5 min after the ionophoresis ended. (F), as (C), showing superimposed cumulative sums of responses from the ipsilateral Q (black) and the contralateral Q (grey). Dotted horizontal line indicates the discrimination level; only spikes crossing this line were used for constructing the histograms.
Effects of NA were investigated in seven neurons with input from contralateral group II afferents, five of which were monosynaptically activated from RF and two monosynaptically activated from ipsilateral group II afferents. In all of these neurons, the modulatory effects of NA on the coQ responses were similar to the effects on responses from RF (facilitated) or from ipsilateral group II afferents (depressed). The depression of responses from coQ was prompt and in both neurons the number of responses was reduced to zero during ionophoresis. In Fig. 5D–F the depression is illustrated with cumulative sums of responses of an interneuron rather than PSTHs because they allow a direct comparison of effects of NA on responses evoked from ipsilateral (black traces) and contralateral (grey traces) group II afferents.
Contacts between monaminergic nerve fibres and commissural interneurons
A total sample of 30 commissural interneurons were labelled, seven of which were selected for detailed morphological analysis. These included two interneurons with input from group II afferent fibres (and no or negligible input from RF), three interneurons with input from RF (and no or negligible input from group II afferents) and two interneurons which were monosynaptically excited from RF and either monosynaptically or disynaptically by group II afferent fibres. Examples of records from neurons of these groups are shown in Fig. 6.
The interneurons were divided into three groups; those with selective input from group II afferents or from the RF, and those with input from both. As shown in Fig. IB, the somata of all of the labelled commissural interneurons were located in lamina VIII or in the adjacent area of lamina VII and the three groups were intermingled within this area. No major differences were found in the extents of their dendritic trees, which extended radially over distances of 570 (240–1200) mediolaterally, 579 (60–1320) dorsoventrally, and 367 (60–500) μm rostrocaudally from the soma (means and ranges for the three groups, respectively). These dendrites extended from the medial to the lateral borders of the grey matter, sometimes reaching into the white matter. The size ranges of the cell bodies of these interneurons [35.3 (20–55), 39 (25–80), 38.3 (30–52.5) μm, mean diameters and range], the surface areas of the cell bodies, and the total dendritic lengths (see Table 1) also overlapped. Another similarity between group II-activated and RF-activated commissural interneurons is the absence of ipsilateral axon collaterals. For further information on morphology of these neurons see Bannatyne et al. 2003.
Table 1.
The numbers and densities of 5-HT and NA terminals in apposition with the cell bodies and dendrites of seven commissural interneurons
| Soma
|
Dendrites
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of contacts
|
Number of contacts
|
Contact density (n/100μm2)
|
Number of contacts
|
Contact density (n/100μm2)
|
||||||||||
| Inputs/Lamina VIII subpopulation | Inter neurons ID | 5HT | NA | 5HT | NA | Surface area2) (μm | 5HT | NA | Total dendritic length (μm) | 5HT | NA | Surface area2) (μm | 5HT | NA |
| Group II, monosynaptic inputs | ||||||||||||||
| Group II | 1 | 83 | 45 | 9 | 2 | 1480.64 | 0.61 | 0.14 | 4841.3 | 74 | 43 | 38299.6 | 0.19 | 0.11 |
| 2 | 203 | 16 | 0 | 0 | 3698.49 | 0 | 0 | 12570.1 | 209 | 16 | 85365.6 | 0.24 | 0.02 | |
| Group II, disynaptic and RF inputs | ||||||||||||||
| Group II and RF | 3 | 86 | 28 | 0 | 0 | 2005.91 | 0 | 0 | 8054.3 | 86 | 28 | 64073.1 | 0.13 | 0.04 |
| 4 | 55 | 2 | 3 | 0 | 5396.92 | 0.02 | 0 | 5336.2 | 52 | 2 | 53977.3 | 0.1 | 0.004 | |
| RF inputs | ||||||||||||||
| RF | 5 | 156 | 7 | 0 | 0 | 1687.16 | 0 | 0 | 9294.8 | 156 | 7 | 45355.6 | 0.34 | 0.02 |
| 6 | 152 | 40 | 3 | 0 | 2600.82 | 0.12 | 0 | 10251.6 | 149 | 40 | 84972.7 | 0.18 | 0.05 | |
| 7 | 63 | 10 | 0 | 0 | 2103.09 | 0 | 0 | 5339.3 | 63 | 10 | 36006.5 | 0.17 | 0.03 | |
| Group II input | ||||||||||||||
| Intermediate zone Group II* | ||||||||||||||
| Mean (SD) | 1–5 | 140 (28) | 37.8 (13) | 6.2 (2.8) | 2 (2.1) | 4769.6 (1926.8) | 0.13 (0.07) | 0.04 (0.03) | 3727.26 (1046.76) | 0.29 (0.05) | 0.08 (0.03) | |||
Contacts for cells 2, 3, 4 and 6 are illustrated in Figs 7 and 8. *Similar data for another population of interneurons with group II input, those located in the intermediate zone and projecting to ipsilateral motor nuclei (Maxwell et al., 2000).
No major differences were found between the relative proportions or the distributions of 5-HT- and NA-immunoreactive nerve terminals in contact with the interneurons with different kinds of input. Figure 7B–H shows examples of contacts of serotoninergic (red) and noradrenergic (green) axon terminals with the dendrites of two labelled interneurons.
Fig. 7.
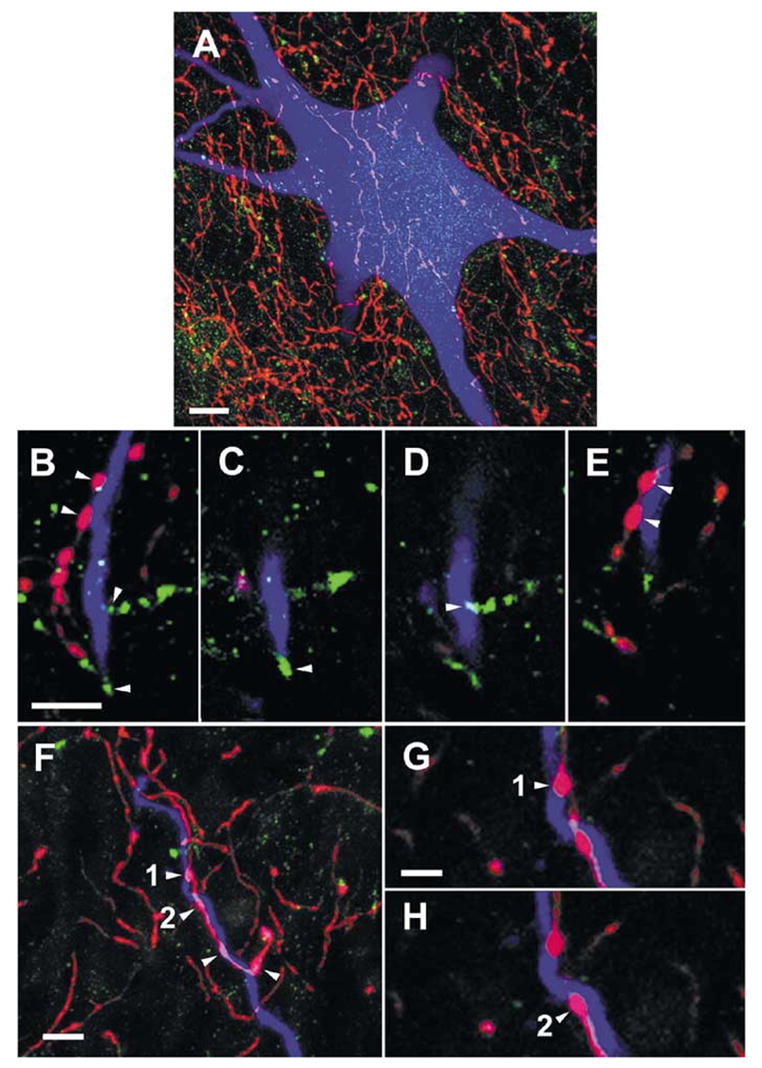
Examples of contacts between monoaminergic fibres and commissural interneurons. (A) A projected image compiled from 85 × 0.5-μm optical sections through the soma of a cell (with RF input; cell 6 in Table 1, records in Fig. 6G-I), showing the abundance of 5-HT-immunoreactive axons bearing varicosities (red) in the vicinity of the cell soma and the relative infrequency of d.b.h.-immunoreactive axons (green) in the same area. (B) A projected image (compiled from 26 × 0.5-μm optical sections) of a dendrite from the cell shown in A. Boutons immunoreactive for 5-HT (shown in red) and d.b.h. (green) can be seen close to the dendrite. (C–E). Single optical sections from the series shown in B illustrate contacts made by the boutons labelled by arrowheads in B onto the dendrite. (F) A further projected image (compiled from 16 × 0.5-μm optical sections) showing a 5-HT-containing axon forming four boutons along a labelled dendrite (originating from a cell with input from both RF and group II afferents; cell 4 in Table 1). (G) and (H) show the terminals labelled 1 and 2 in (F) at higher magnification. Scale bars, 10 μm (A), 5 μm (B–F), 2.5 μm (G–H).
Distributions of 5-HT- and NA-immunoreactive terminals in contact with three different interneurons, representing commissural interneurons with input from group II afferents, from RF and from both group II and RF are illustrated in Fig. 8A, D and E. The histograms in Fig. 8B, C and G show the numbers of contacts at different distances from the soma (Sholl analysis) for the three cells, illustrating in a quantitative way that both 5-HT- and NA-immunoreactive terminals made contacts over the entire dendritic tree of these neurons. In four other cells analysed in the same way 5-HT- and NA-immunoreactive terminals were distributed similarly over the dendritic trees (see Table 1).
Fig. 8.
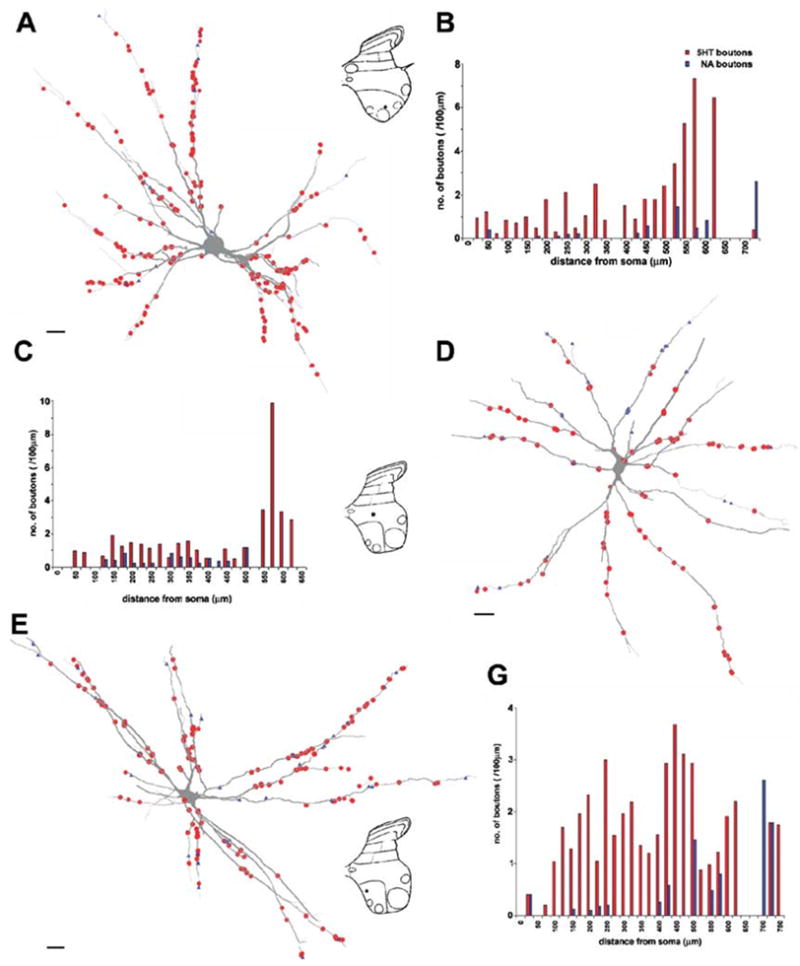
Distribution of 5-HT- and NA-immunoreactive terminals on three commissural interneurons. (A, D and E) Reconstructions of the dendritic trees of three commissural interneurons, with input from group II afferents, from RF and from both (nos 2, 6 and 3, respectively, in Table 1) and distributions of 5-HT (red circles) and d.b.h. (blue triangles) contacts throughout their dendritic trees. Insets show the positions of cell bodies within spinal grey matter. (B, C and G) Sholl plots for the two types of varicosity showing the distribution (number per 100 μm dendritic length) of contacts at 25-μm intervals throughout the dendritic tree. Data sets in red represent 5HT-immunoreactive boutons, in blue d.b.h.-immunopositive boutons. Records from these cells are shown in Fig. 6A–C, D–F and H and I, respectively. Scale bars, 20 μm.
Table 1 summarizes the morphological data, showing that the number of 5-HT terminals in contact with dendrites exceeded the number of NA terminals in all of the seven neurons. In addition, in all neurons 5-HT-immunoreactive axons could be observed running parallel with labelled dendrites and forming several appositions, while only single NA-containing varicosities were found in contact with either cell bodies or dendrites.
Discussion
These results extend previous observations on the monoaminergic modulation of transmission from peripheral afferents to spinal interneurons (Bras et al., 1989; Jankowska et al., 2000; Hammar & Jankowska, 2003) and reveal modulation of transmission between descending fibres from the reticular formation to lumbar commissural interneurons. They show that both 5-HT and NA potently modulate the activation of lamina VIII commissural interneurons and that activation of commissural interneurons from group II muscle afferents and from the reticular formation is differently affected by NA. This indicates not only the importance of monoamines for modifying motor outputs that engage both halves of the spinal cord, but also that reflex movements and centrally initiated movements sources may be modulated differently, dependent on context.
Differential 5-HT and NA modulatory actions
The different patterns of modulation seen to responses evoked from the RF and from group II afferents by NA and 5-HT are striking. Two possible mechanisms might be considered. The first is that monoamines modulate transmission from group II afferents and from descending reticulospinal tract fibres to commissural interneurons differently, acting presynaptically on the terminals of group II afferents and reticulospinal neurons. However, responses were rarely evoked from both of these sources in any single commissural neuron, so a second possibility is that the monoamines may have different post-synaptic effects on different subpopulations of commissural interneurons.
At first glance, the commissural interneurons had dominant input from either group II afferents or RF fibres. In extracellular records, commissural neurons were only discharged by one of these inputs (and could hence be tested with ionophoresis only for that input). Closer inspection of the responses in intracellular recordings revealed, however, that in a small proportion of the interneurons both group II afferents and RF fibres evoked responses at short latency. For example some of the commissural interneurons in which large monosynaptic EPSPs were evoked by RF stimulation also had smaller EPSPs from group II muscle afferents, and vice versa. In most cases it was difficult to determine whether the smaller additional EPSPs were evoked mono-or disynaptically. It was also difficult to exclude the possibility that these neurons also had input from other sources that were not tested (e.g. from group II afferents of iliopsoas; Aggelopoulos et al., 1996a). The subdivision of lamina VIII interneurons into three subpopulations reflects the input patterns we have seen. However, these commissural interneurons might also be considered to constitute one population of interneurons, in which input from group II afferents and descending tract neurons (reticulospinal as well as vestibulospinal; see Krutki et al., 2003) is distributed to individual interneurons in different combinations. The latter organization would resemble distribution of inputs to interneurons in pathways from group I afferents (Harrison & Jankowska, 1985) and from group II afferents (Edgley, 2001). The similar morphological characteristics of the commissural interneurons with input from group II afferents and those with input from RF would be consistent with this idea. As shown in Results, the locations, soma sizes, extents of dendritic trees, axonal projections and distributions of immunoreactive 5-HT and NA axon terminals were similar for the neurons with different input patterns.
If distinct subpopulations of commissural neurons expressed different subclasses of NA receptors, the different effects of NA on responses evoked from RF and from group II afferents could be explained by different postsynaptic modulatory actions. The similarity between the effects of 5-HT and NA on responses evoked from contralateral group II afferents, both in neurons with input from ipsilateral group II afferents (which were depressed) and in neurons with input from the RF (which were facilitated) suggests that the actions of NA are generally postsynaptic, because NA was applied ionophoretically close to the commissural interneuron on one side of the spinal cord and could therefore not act directly on the terminals of contralateral group II afferents, which were on the other side of the spinal cord. The possibility that the actions were predominantly postsynaptic is also supported by the analysis of the distribution of immunoreactive 5-HT and NA axon terminals, both of which have been found to make contacts with commissural interneurons, with similar distributions. The presence of these contacts indicates that at least some of the observed effects of monoamines are exerted postsynaptically. Postsynaptic actions could be mediated not only by the considerable number of 5-HT contacts but also by the scarcer NA contacts, especially as postsynaptic actions of NA could also be evoked by volume transmission (see, e.g., Ridet et al., 1993).
However, the fact that responses evoked from group II afferents were depressed while responses evoked from RF were facilitated by NA is difficult to reconcile with different postsynaptic actions on individual interneurons within a single neuronal population. An alternative explanation might therefore be that NA facilitates activation of all commissural interneurons by postsynaptic actions but these postsynaptic actions (resulting in enhancement of responses of RF origin) are associated with strong presynaptic depression of transmission from group II afferents. The depression of polysynaptic actions from contralateral group II afferents by NA, could likewise be caused by presynaptic actions on the terminals of spinal interneurons that mediate them.
Previously it was reported that, although the overall effect of noradrenaline was facilitation of transmission from group I afferents, opposing effects, mediated by different subclasses of receptors, were found on interneurons in pathways from group I afferents (Hammar & Jankowska, 2003) and on ventral spinocerebellar tract neurons (Hammar et al., 2002) where α1 and β receptor agonists facilitated and α2 receptor agonists depressed responses. In keeping with the possibility of presynaptic depressive actions via α2 receptors, α2 receptors have been reported on some primary afferent terminals containing substance P (Stone et al., 1998) and mRNA for α2 receptors has been found in cells in dorsal root ganglia (Nicholas et al., 1993; Gold et al., 1997). Although morphological studies on feline spinal cord have failed to reveal axoaxonic contacts between NA-containing axons and primary afferent terminals (Maxwell & Bannatyne, 1983; Doyle & Maxwell, 1991) the depressive presynaptic actions of NA on group II mediated responses might be exerted via volume conductance.
Lack of relationship between the distribution of monoaminergic contacts and actions of monoamines
Because NA had opposite effects on responses evoked by RF fibres and by group II afferents, the question arose of whether this might be related to different relationships between NA-releasing fibres and commissural interneurons with different inputs. However, no major differences were found between the different groups of neurons. Both the total numbers and densities of d.b.h.-immunoreactive contacts on dendrites and cell bodies were within the same range, and the distributions of contacts along the dendritic trees were similar.
Previous morphological studies on ipsilaterally projecting intermediate zone premotor interneurons with input from group II muscle afferents (Jankowska et al., 1997; Maxwell et al., 2000) revealed a similar pattern of monoaminergic contacts to that found in commissural interneurons. Data for the ipsilaterally projecting intermediate zone interneurons are included at the bottom of Table 1 for comparison. In both cases the average number of serotoninergic contacts was at least three times higher than that of noradrenergic contacts, and appositions were mainly made on dendrites where they were distributed along the dendritic tree, whereas none or only very few contacts were found on the soma. Again, these similarities did not match the differences in the modulatory effects of monoamines upon the ipsilaterally projecting intermediate zone interneurons (which were depressed by NA and either facilitated or depressed by 5-HT (Jankowska et al., 2000). Taken together, these studies indicate that differences in modulatory actions of monoamines are unlikely to be reflected in the gross morphology or distribution of contacts, but more probably reflect the existence of different pre- and/or postsynaptic membrane receptors which determine the ways that these neurons respond.
Functional considerations
In several studies, especially those on highly reduced in vitro preparations, the effects of monoamines are primarily considered with respect to the activity of spinal neurons related to rhythmic motor activities such as locomotion or scratching (Cazalets et al., 1990; Barbeau & Rossignol, 1991; Cazalets et al., 1992; Kiehn et al., 1999; Schmidt & Jordan, 2000). Previous observations on commissural interneurons activated by reticulospinal tract fibres likewise related their activity to locomotion (Matsuyama & Mori, 1998; Mori et al., 1998) and this possibility has also been suggested with respect to commissural interneurons with group II input (Jankowska & Noga, 1990). The modulatory actions of monoamines on commissural interneurons might thus be considered in terms of assisting either the initiation or the control of locomotion. If so, the depression of activation of commissural interneurons from group II afferents by NA could represent another example of the weakening of reflex actions of group II muscle afferents during locomotion (Shefchyk et al., 1990; Perreault et al., 1995; Perreault et al., 1999) and facilitation of their activation from RF as an expression of strengthening of the central drive via these neurons. In relation to the potential role of these neurons in locomotion, Rossignol and colleagues have shown that manipulation of noradrenergic receptors intraspinally in the same segments that contain the commissural neurons can powerfully influence the expression of locomotor activity: the α2 agonist clonidine promotes locomotor activity and the α2 antagonist yohimbine depresses locomotor activity (Marcoux & Rossignol, 2000; Rossignol et al., 2002).
However, there are no reasons for linking activity of these commissural interneurons to only one kind of motor activity, because all known spinal neurons subserve a variety of centrally or reflexly initiated reactions (see Jankowska, 2001) Serotonin-releasing neurons could increase the probability of activation of commissural neurons involved in any movements that require coordinated activity of left and right limbs. Noradrenaline releasing neurons might on the other hand be more specifically involved in adjusting the operation of neuronal networks that are activated by muscle spindle afferents, either rhythmic, phasic or tonic, and in ensuring an appropriate degree of contraction following muscle stretch. Even though the effects of serotonin on various subpopulations of commissural interneurons appears to be less differentiated, differentiated effects may be induced by changing the balance between the direct actions of commissural interneurons and indirect actions mediated via other interneurons. An interesting example has been recently reported by Butt & Kiehn (2003) who showed that polysynaptic inhibition of contralateral motoneurons by commissural interneurons in neonatal rats may be replaced by mono-synaptic excitation when the actions of the interneurons mediating the inhibition are eliminated by 5-HT.
A change in the balance between excitatory and inhibitory actions of commissural interneurons may be one of the main mechanisms behind the selection between the various patterns of crossed actions of group II afferents. As shown by Aggelopoulos and coworkers (Aggelopoulos et al., 1996b), activity of descending serotoninergic pathways is required for the expression of crossed inhibition of group II origin (see Introduction). The facilitation of responses of commissural interneurons evoked by group II afferents by ionophoretically applied 5-HT is entirely in keeping with this mechanism. However, because the effects of 5-HT on all of the commissural neurons tested were so consistent, and because both excitatory or inhibitory commissural neurons exist (Bannatyne et al., 2003), 5-HT should facilitate activation of both excitatory and inhibitory commissural neurons. For differential modulation of actions of the excitatory and inhibitory subpopulations of these neurons it would therefore be particularly interesting if the parallel depressive actions of NA were primarily targeted to inhibitory commissural interneurons with dominant group II input whereas the excitatory commissural interneurons were coexcited by reticulospinal tract fibres and therefore likely to be more effectively activated.
Furthermore, it should be borne in mind that transmission from group II afferents is modulated not only by monoamines but also by the presynaptic actions of GABAergic interneurons with input from group II and cutaneous afferents, which are themselves also under monoaminergic control. Monoamines might thus have additional effects by either enhancing or weakening presynaptic inhibition at the level of synapses between group II afferents and commissural interneurons (Riddell et al., 1993; Riddell et al., 1995; Maxwell & Riddell, 1999) or at the level of intervening neurons in parallel pathways (Jankowska et al., 2002a; Edgley et al., 2003). The selection and modulation of reflex actions from group II muscle afferents will thus depend on actions of monoamines at multiple sites within the pathways from these afferents.
Acknowledgments
We wish to thank Rauni Larsson, Margaret McGill and Robert Kerr for their invaluable assistance. The study was supported by a National Institutes of Health Grant NS 40 863.
Abbreviations
- 5-HT
5-hydroxytryptamine, serotonin
- coQ
contralateral quadriceps nerve
- d.b.h.
dopamine-beta-hydroxylase
- DP
deep peroneal nerve
- GS
gastrocnemius-soleus nerve
- L
lumbar segment
- MLF
medial longitudinal fasciculus
- NA
noradrenaline
- PSTH
peri-stimulus time histogram
- Q
quadriceps nerve
- RF
reticular formation
- S
sacral segment
- Sart
sartorius nerve
- Th
thoracic segment
References
- Aggelopoulos NC, Bawa P, Edgley SA. Activation of midlumbar neurones by afferents from anterior hindlimb muscles in the cat. J Physiol (Lond) 1996a;497:795–802. doi: 10.1113/jphysiol.1996.sp021810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci. 1996b;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggelopoulos NC, Edgley SA. Segmental localisation of the relays mediating crossed inhibition of hindlimb motoneurones from group II afferents in the anaesthetized cat spinal cord. Neurosci Lett. 1995;185:60–64. doi: 10.1016/0304-3940(94)11225-8. [DOI] [PubMed] [Google Scholar]
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol (Lond) 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell D. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, McCrea D. Comparison of effects of monoamines on transmission in spinal pathways from group I and II muscle afferents in the cat. Exp Brain Res. 1989;76:27–37. doi: 10.1007/BF00253620. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left–right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Cajal S. Histologie Du Systeme Nerveux de L'homme and Des Vertebres. Institute Ramon y Cajal; Madrid, Spain: 1953. [Google Scholar]
- Cazalets JR, Grillner P, Menard I, Cremieux J, Clarac F. Two types of motor rhythm induced by NMDA and amines in an in vitro spinal cord preparation of neonatal rat. Neurosci Lett. 1990;111:116–121. doi: 10.1016/0304-3940(90)90354-c. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol (Lond) 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle CA, Maxwell DJ. Catecholaminergic innervation of the spinal dorsal horn: a correlated light and electron microscopic analysis of tyrosine hydroxylase–immunoreactive fibres in the cat. Neuroscience. 1991;45:161–176. doi: 10.1016/0306-4522(91)90112-2. [DOI] [PubMed] [Google Scholar]
- Edgley SA. Organisation of inputs to spinal interneurone populations. J Physiol (Lond) 2001;533:51–56. doi: 10.1111/j.1469-7793.2001.0051b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol (Lond) 1987;385:393–13. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Krutki P, Hammar I. Both dorsal horn and lamina viii interneurones contribute to crossed reflexes from feline group ii muscle afferents. J Physiol (Lond) 2003;552:961–974. doi: 10.1113/jphysiol.2003.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I, Källström Y, Marshall KC. Double micromanipulator for independant impalements of one neurone with two electrodes. Acta Physiol Scand. 1972;84:4A. [Google Scholar]
- Gauthier L, Rossignol S. Contralateral hindlimb responses to cutaneous stimulation during locomotion in high decerebrate cats. Brain Res. 1981;207:303–320. doi: 10.1016/0006-8993(81)90366-8. [DOI] [PubMed] [Google Scholar]
- Gold MS, Dastmalchi S, Levine JD. Alpha 2-adrenergic receptor subtypes in rat dorsal root and superior cervical ganglion neurons. Pain. 1997;69:179–190. doi: 10.1016/s0304-3959(96)03218-6. [DOI] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. Contralateral reflex reversal controlled by limb position in the acute spinal cat injected with clonidine i.v. Brain Res. 1978;144:411–14. doi: 10.1016/0006-8993(78)90169-5. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct andindirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol (Lond) 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Chojnicka B, Jankowska E. Modulation of responses of feline ventral spinocerebellar tract neurons by monoamines. J Comp Neurol. 2002;443:298–309. doi: 10.1002/cne.10135. [DOI] [PubMed] [Google Scholar]
- Hammar I, Jankowska E. Modulatory effects of alpha1-, alpha2-, and beta-receptor agonists on feline spinal interneurons with monosynaptic input from group I muscle afferents. J Neurosci. 2003;23:332–338. doi: 10.1523/JNEUROSCI.23-01-00332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol (Lond) 1985;361:403–18. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E, Zytnicki D. Lamina VIII interneurones interposed in crossed reflex pathways in the cat. J Physiol (Lond) 1986;371:147–166. doi: 10.1113/jphysiol.1986.sp015965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB. Some methods for selective activation of muscle afferent fibres. In: Porter R, editor. Studies in Neurophysiology. University Press; Cambridge: 1978. pp. 155–176. [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol (Lond) 2001;533:31–40. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/ or group II muscle afferents. Eur J Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Maxwell DJ, Dolk S, Dahlstrom A. A confocal and electron microscopic study of contacts between 5-HT fibres and feline dorsal horn interneurons in pathways from muscle afferents. J Comp Neurol. 1997;387:430–438. [PubMed] [Google Scholar]
- Jankowska E, Noga BR. Contralaterally projecting lamina VIII interneurones in middle lumbar segments in the cat. Brain Res. 1990;535:327–330. doi: 10.1016/0006-8993(90)91618-q. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. Differential presynaptic inhibition of actions of group II afferents in di- and polysynaptic pathways to feline motoneurones. J Physiol (Lond) 2002a;542:287–299. doi: 10.1113/jphysiol.2001.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. On organization of a neuronal network in pathways from group II muscle afferents in feline lumbar spinal segments. J Physiol (Lond) 2002b;542:301–314. doi: 10.1113/jphysiol.2001.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Sillar KT, Kjaerulff O, McDearmid JR. Effects of noradrenaline on locomotor rhythm-generating networks in the isolated neonatal rat spinal cord. J Neurophysiol. 1999;82:741–746. doi: 10.1152/jn.1999.82.2.741. [DOI] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci. 2003;23:8041–8050. doi: 10.1523/JNEUROSCI.23-22-08041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoux J, Rossignol S. Initiating or blocking locomotion in spinal cats by applying noradrenergic drugs to restricted lumbar spinal segments. J Neurosci. 2000;20:8577–8585. doi: 10.1523/JNEUROSCI.20-22-08577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Mori S. Lumbar interneurons involved in the generation of fictive locomotion in cats. Ann NY Acad Sci. 1998;860:441–443. doi: 10.1111/j.1749-6632.1998.tb09070.x. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Bannatyne BA. Ultrastructure of muscle spindle afferent terminations in lamina VI of the cat spinal cord. Brain Res. 1983;288:297–301. doi: 10.1016/0006-8993(83)90106-3. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Riddell JS. Axoaxonic synapses on terminals of group II muscle spindle afferent axons in the spinal cord of the cat. Eur J Neurosci. 1999;11:2151–2159. doi: 10.1046/j.1460-9568.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Riddell JS, Jankowska E. Serotoninergic and noradrenergic axonal contacts associated with premotor interneurons in spinal pathways from group II muscle afferents. Eur J Neurosci. 2000;12:1271–1280. doi: 10.1046/j.1460-9568.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K. Cerebellar-induced locomotion: reticulospinal control of spinal rhythm generating mechanism in cats. Ann NY Acad Sci. 1998;860:94–105. doi: 10.1111/j.1749-6632.1998.tb09041.x. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone V, Hokfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Rossignol S. Fictive motor patterns in chronic spinal cats. J Neurophysiol. 1991;66:1874–1887. doi: 10.1152/jn.1991.66.6.1874. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol (Lond) 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M, Shefchyk SJ, Jimenez I, McCrea DA. Depression of muscle and cutaneous afferent–evoked monosynaptic field potentials during fictive locomotion in the cat. J Physiol (Lond) 1999;521:691–703. doi: 10.1111/j.1469-7793.1999.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Eide E. Depolarization of group II muscle afferents by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol (Lond) 1993;461:723–741. doi: 10.1113/jphysiol.1993.sp019538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Huber J. Organization of neuronal systems mediating presynaptic inhibition of group II muscle afferents in the cat. J Physiol (Lond) 1995;483:443–60. doi: 10.1113/jphysiol.1995.sp020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Rajaofetra N, Teilhac JR, Geffard M, Privat A. Evidence for nonsynaptic serotonergic and noradrenergic innervation of the rat dorsal horn and possible involvement of neuron–glia interactions. Neuroscience. 1993;52:143–157. doi: 10.1016/0306-4522(93)90189-m. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Bouyer L, Barthelemy D, Langlet C, Leblond H. Recovery of locomotion in the cat following spinal cord lesions. Brain Res Brain Res Rev. 2002;40:257–266. doi: 10.1016/s0165-0173(02)00208-4. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Gauthier L. An analysis of mechanisms controlling the reversal of crossed spinal reflexes. Brain Res. 1980;182:31–5. doi: 10.1016/0006-8993(80)90828-8. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Julien C, Gauthier L. Stimulus–response relationships during locomotion. Can J Physiol Pharmacol. 1981;59:667–674. doi: 10.1139/y81-102. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Spinal motorneurons, interneurons and Renshaw cells. A Golgi study. Arch Ital Biol. 1966;104:328–353. [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, McCrea D, Kriellaars D, Fortier P, Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Exp Brain Res. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Sherrington C. The Integrative Action of the Nervous System. Yale University Press; New Haven and London: 1906. [Google Scholar]
- Stokke MF, Nissen UV, Glover JC, Kiehn O. Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J Comp Neurol. 2002;446:349–359. doi: 10.1002/cne.10211. [DOI] [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


