Abstract
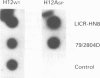
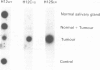
Activation of the ras gene family by point mutation at codons 12, 13 and 61 has been demonstrated in up to 20% of unselected series of human tumours. The present study was carried out to assess the incidence of ras activation in 37 squamous cell carcinomas of the head and neck, seven squamous cell carcinomas of the skin and eight squamous carcinoma cell lines. Oligonucleotide probes and the polymerase chain reaction were used on DNA extracted from achival paraffin embedded material. Mutations in codon 12 of the Harvey ras gene was found in a carcinoma of the larynx and a carcinoma of the lip, both of which had received prior irradiation. A cell line (LICR-LON-HN8) established from the same laryngeal cancer showed the same mutation. This study indicates that there is a low incidence of ras mutation in human squamous cell carcinomas and that activation of this family of genes is probably not a common factor in the development of this group of tumours.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Ananthaswamy H. N., Price J. E., Goldberg L. H., Bales E. S. Detection and identification of activated oncogenes in human skin cancers occurring on sun-exposed body sites. Cancer Res. 1988 Jun 15;48(12):3341–3346. [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L. The ras gene family and human carcinogenesis. Mutat Res. 1988 May;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Burman J. F., Carter R. L. Lysis of type-I collagen by squamous carcinomas of the head and neck. Int J Cancer. 1985 Jul 15;36(1):109–116. doi: 10.1002/ijc.2910360117. [DOI] [PubMed] [Google Scholar]
- Carter R. L., Burman J. F., Barr L., Gusterson B. A. Immunohistochemical localization of basement membrane type IV collagen in invasive and metastatic squamous carcinomas of the head and neck. J Pathol. 1985 Nov;147(3):159–164. doi: 10.1002/path.1711470303. [DOI] [PubMed] [Google Scholar]
- Carter R. L. Patterns and mechanisms of localized bone invasion by tumors: studies with squamous carcinomas of the head and neck. Crit Rev Clin Lab Sci. 1985;22(3):275–315. doi: 10.3109/10408368509165845. [DOI] [PubMed] [Google Scholar]
- Cowley G., Smith J. A., Ellison M., Gusterson B. Production of beta-human chorionic gonadotrophin by human squamous carcinoma cell lines. Int J Cancer. 1985 May 15;35(5):575–579. doi: 10.1002/ijc.2910350502. [DOI] [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Butler L. J. Ten human carcinoma cell lines derived from squamous carcinomas of the head and neck. Br J Cancer. 1981 Jun;43(6):772–785. doi: 10.1038/bjc.1981.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Pittam M. R., James T. Five human tumour cell lines derived from a primary squamous carcinoma of the tongue, two subsequent local recurrences and two nodal metastases. Br J Cancer. 1981 Sep;44(3):363–370. doi: 10.1038/bjc.1981.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr C. J., Saiki R. K., Erlich H. A., McCormick F., Marshall C. J. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1629–1633. doi: 10.1073/pnas.85.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson B., Cowley G., Smith J. A., Ozanne B. Cellular localisation of human epidermal growth factor receptor. Cell Biol Int Rep. 1984 Aug;8(8):649–658. doi: 10.1016/0309-1651(84)90045-6. [DOI] [PubMed] [Google Scholar]
- Impraim C. C., Saiki R. K., Erlich H. A., Teplitz R. L. Analysis of DNA extracted from formalin-fixed, paraffin-embedded tissues by enzymatic amplification and hybridization with sequence-specific oligonucleotides. Biochem Biophys Res Commun. 1987 Feb 13;142(3):710–716. doi: 10.1016/0006-291x(87)91472-0. [DOI] [PubMed] [Google Scholar]
- Lemoine N. R., Mayall E. S., Wyllie F. S., Williams E. D., Goyns M., Stringer B., Wynford-Thomas D. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene. 1989 Feb;4(2):159–164. [PubMed] [Google Scholar]
- Leon J., Kamino H., Steinberg J. J., Pellicer A. H-ras activation in benign and self-regressing skin tumors (keratoacanthomas) in both humans and an animal model system. Mol Cell Biol. 1988 Feb;8(2):786–793. doi: 10.1128/mcb.8.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Richards C. S., Hendler F., Burns D., Gusterson B. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J Pathol. 1986 May;149(1):9–14. doi: 10.1002/path.1711490104. [DOI] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Rayter Z., McIlhinney R., Gusterson B. Expression of membrane glycoproteins in normal keratinocytes and squamous carcinoma cell lines. Exp Cell Res. 1989 Aug;183(2):443–452. doi: 10.1016/0014-4827(89)90403-5. [DOI] [PubMed] [Google Scholar]
- Rodenhuis S., van de Wetering M. L., Mooi W. J., Evers S. G., van Zandwijk N., Bos J. L. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987 Oct 8;317(15):929–935. doi: 10.1056/NEJM198710083171504. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sawey M. J., Hood A. T., Burns F. J., Garte S. J. Activation of c-myc and c-K-ras oncogenes in primary rat tumors induced by ionizing radiation. Mol Cell Biol. 1987 Feb;7(2):932–935. doi: 10.1128/mcb.7.2.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Martin W. J., Arnheim N. Analysis of DNA sequences in forty-year-old paraffin-embedded thin-tissue sections: a bridge between molecular biology and classical histology. Cancer Res. 1988 Aug 15;48(16):4564–4566. [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]