Abstract
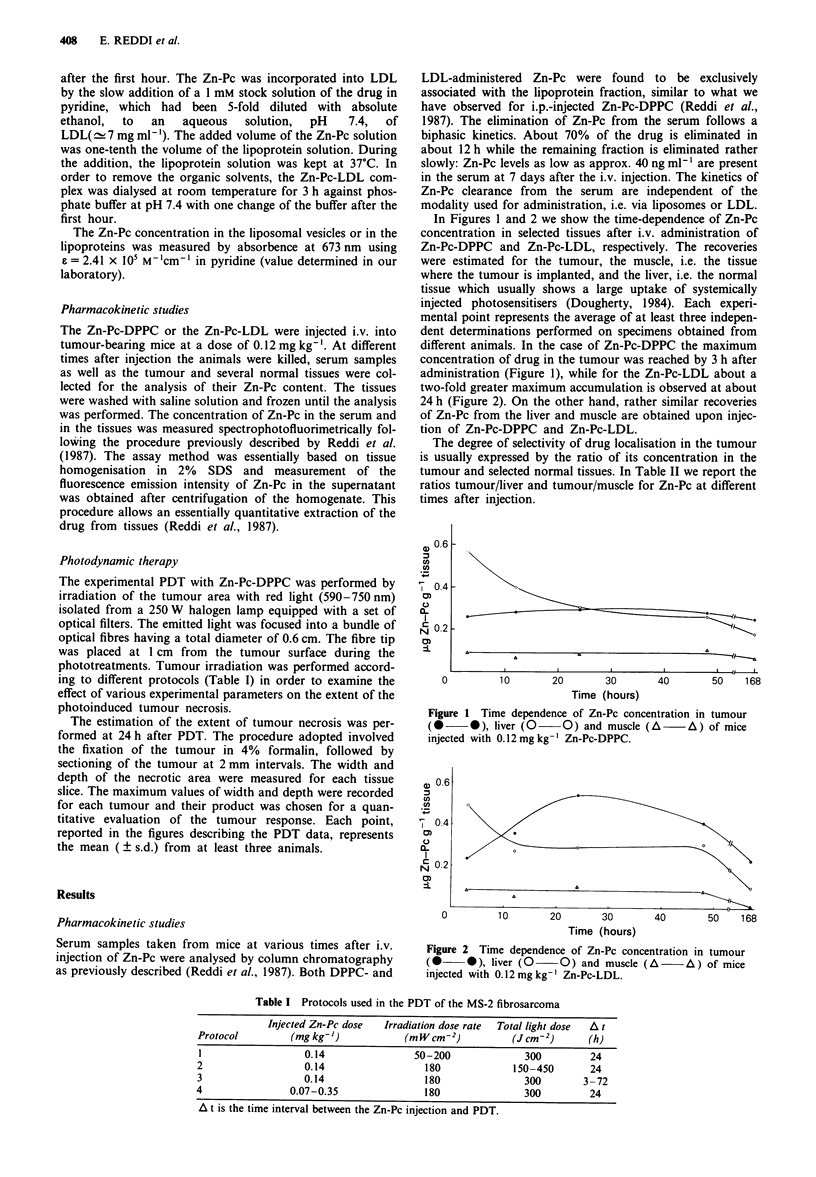
The pharmacokinetics of Zn-phthalocyanine (Zn-Pc) in mice bearing a transplanted MS-2 fibrosarcoma has been studied using dipalmitoyl-phosphatidylcholine (DPPC) liposomes and low density lipoproteins (LDL) as drug delivery systems. LDL induce a higher Zn-Pc uptake by the tumour and improve the selectivity of tumour targeting as compared to DPPC liposomes. Experimental photodynamic therapy (PDT) of the MS-2 fibrosarcoma has been performed using liposome-delivered Zn-Pc and the efficiency of tumour necrosis has been measured following four different irradiation protocols. We found that Zn-Pc doses as low as 0.07-0.35 mg kg-1 are sufficient for inducing an efficient tumour response that is linearly dependent on the injected dose. The amount of Zn-Pc in the tumour decreases very slowly as a function of time, hence PDT gives satisfactory results even if performed at relatively long time intervals after administration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Hur E., Rosenthal I. The phthalocyanines: a new class of mammalian cells photosensitizers with a potential for cancer phototherapy. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):145–147. doi: 10.1080/09553008514550211. [DOI] [PubMed] [Google Scholar]
- Brasseur N., Ali H., Langlois R., Wagner J. R., Rousseau J., van Lier J. E. Biological activities of phthalocyanines--V. Photodynamic therapy of EMT-6 mammary tumors in mice with sulfonated phthalocyanines. Photochem Photobiol. 1987 May;45(5):581–586. doi: 10.1111/j.1751-1097.1987.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Bugelski P. J., Porter C. W., Dougherty T. J. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981 Nov;41(11 Pt 1):4606–4612. [PubMed] [Google Scholar]
- Candide C., Morlière P., Mazière J. C., Goldstein S., Santus R., Dubertret L., Reyftmann J. P., Polonovski J. In vitro interaction of the photoactive anticancer porphyrin derivative photofrin II with low density lipoprotein, and its delivery to cultured human fibroblasts. FEBS Lett. 1986 Oct 20;207(1):133–138. doi: 10.1016/0014-5793(86)80026-6. [DOI] [PubMed] [Google Scholar]
- Chan W. S., Svensen R., Phillips D., Hart I. R. Cell uptake, distribution and response to aluminium chloro sulphonated phthalocyanine, a potential anti-tumour photosensitizer. Br J Cancer. 1986 Feb;53(2):255–263. doi: 10.1038/bjc.1986.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J. Photodynamic therapy (PDT) of malignant tumors. Crit Rev Oncol Hematol. 1984;2(2):83–116. doi: 10.1016/s1040-8428(84)80016-5. [DOI] [PubMed] [Google Scholar]
- Fingar V. H., Potter W. R., Henderson B. W. Drug and light dose dependence of photodynamic therapy: a study of tumor cell clonogenicity and histologic changes. Photochem Photobiol. 1987 May;45(5):643–650. doi: 10.1111/j.1751-1097.1987.tb07392.x. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayata Y., Kato H., Konaka C., Ono J., Takizawa N. Hematoporphyrin derivative and laser photoradiation in the treatment of lung cancer. Chest. 1982 Mar;81(3):269–277. doi: 10.1378/chest.81.3.269. [DOI] [PubMed] [Google Scholar]
- Kessel D. Proposed structure of the tumor-localizing fraction of HPD (hematoporphyrin derivative). Photochem Photobiol. 1986 Aug;44(2):193–196. doi: 10.1111/j.1751-1097.1986.tb03585.x. [DOI] [PubMed] [Google Scholar]
- Kessel D. Sites of photosensitization by derivatives of hematoporphyrin. Photochem Photobiol. 1986 Oct;44(4):489–493. doi: 10.1111/j.1751-1097.1986.tb04697.x. [DOI] [PubMed] [Google Scholar]
- Kessel D., Thompson P., Saatio K., Nantwi K. D. Tumor localization and photosensitization by sulfonated derivatives of tetraphenylporphine. Photochem Photobiol. 1987 Jun;45(6):787–790. doi: 10.1111/j.1751-1097.1987.tb07883.x. [DOI] [PubMed] [Google Scholar]
- Langlois R., Ali H., Brasseur N., Wagner J. R., van Lier J. E. Biological activities of phythalocyanines--IV. Type II sensitized photooxidation of L-tryptophan and cholesterol by sulfonated metallo phthalocyanines. Photochem Photobiol. 1986 Aug 2;44(2):117–123. doi: 10.1111/j.1751-1097.1986.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Milanesi C., Biolo R., Reddi E., Jori G. Ultrastructural studies on the mechanism of the photodynamic therapy of tumors. Photochem Photobiol. 1987 Nov;46(5):675–681. doi: 10.1111/j.1751-1097.1987.tb04831.x. [DOI] [PubMed] [Google Scholar]
- Reddi E., Lo Castro G., Biolo R., Jori G. Pharmacokinetic studies with zinc(II)-phthalocyanine in tumour-bearing mice. Br J Cancer. 1987 Nov;56(5):597–600. doi: 10.1038/bjc.1987.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau J., Ali H., Lamoureux G., Lebel E., van Lier J. E. Synthesis, tissue distribution and tumor uptake of 99mTc- and 67Ga-tetrasulfophthalocyanine. Int J Appl Radiat Isot. 1985 Sep;36(9):709–716. doi: 10.1016/0020-708x(85)90041-9. [DOI] [PubMed] [Google Scholar]
- Shumaker B. P., Hetzel F. W. Clinical laser photodynamic therapy in the treatment of bladder carcinoma. Photochem Photobiol. 1987 Nov;46(5):899–901. doi: 10.1111/j.1751-1097.1987.tb04866.x. [DOI] [PubMed] [Google Scholar]
- Spikes J. D. Phthalocyanines as photosensitizers in biological systems and for the photodynamic therapy of tumors. Photochem Photobiol. 1986 Jun;43(6):691–699. doi: 10.1111/j.1751-1097.1986.tb05648.x. [DOI] [PubMed] [Google Scholar]
- Tralau C. J., Barr H., Sandeman D. R., Barton T., Lewin M. R., Bown S. G. Aluminum sulfonated phthalocyanine distribution in rodent tumors of the colon, brain and pancreas. Photochem Photobiol. 1987 Nov;46(5):777–781. doi: 10.1111/j.1751-1097.1987.tb04847.x. [DOI] [PubMed] [Google Scholar]
- Tralau C. J., MacRobert A. J., Coleridge-Smith P. D., Barr H., Bown S. G. Photodynamic therapy with phthalocyanine sensitisation: quantitative studies in a transplantable rat fibrosarcoma. Br J Cancer. 1987 Apr;55(4):389–395. doi: 10.1038/bjc.1987.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valduga G., Reddi E., Jori G. Spectroscopic studies on Zn(II)-phthalocyanine in homogeneous and microheterogeneous systems. J Inorg Biochem. 1987 Jan;29(1):59–65. doi: 10.1016/0162-0134(87)80012-0. [DOI] [PubMed] [Google Scholar]
- Vitols S. G., Masquelier M., Peterson C. O. Selective uptake of a toxic lipophilic anthracycline derivative by the low-density lipoprotein receptor pathway in cultured fibroblasts. J Med Chem. 1985 Apr;28(4):451–454. doi: 10.1021/jm00382a011. [DOI] [PubMed] [Google Scholar]
- Zalar G. L., Poh-Fitzpatrick M., Krohn D. L., Jacobs R., Harber L. C. Induction of drug photosensitization in man after parenteral exposure to hematoporphyrin. Arch Dermatol. 1977 Oct;113(10):1392–1397. [PubMed] [Google Scholar]


