Abstract
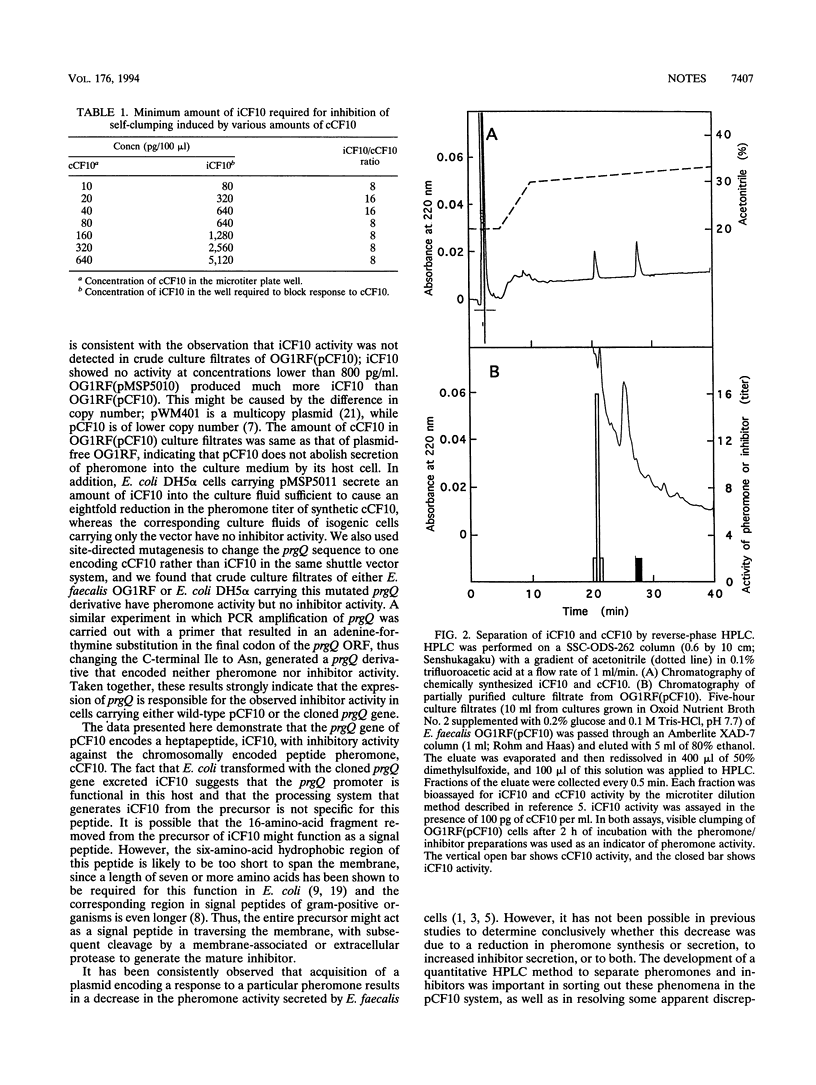
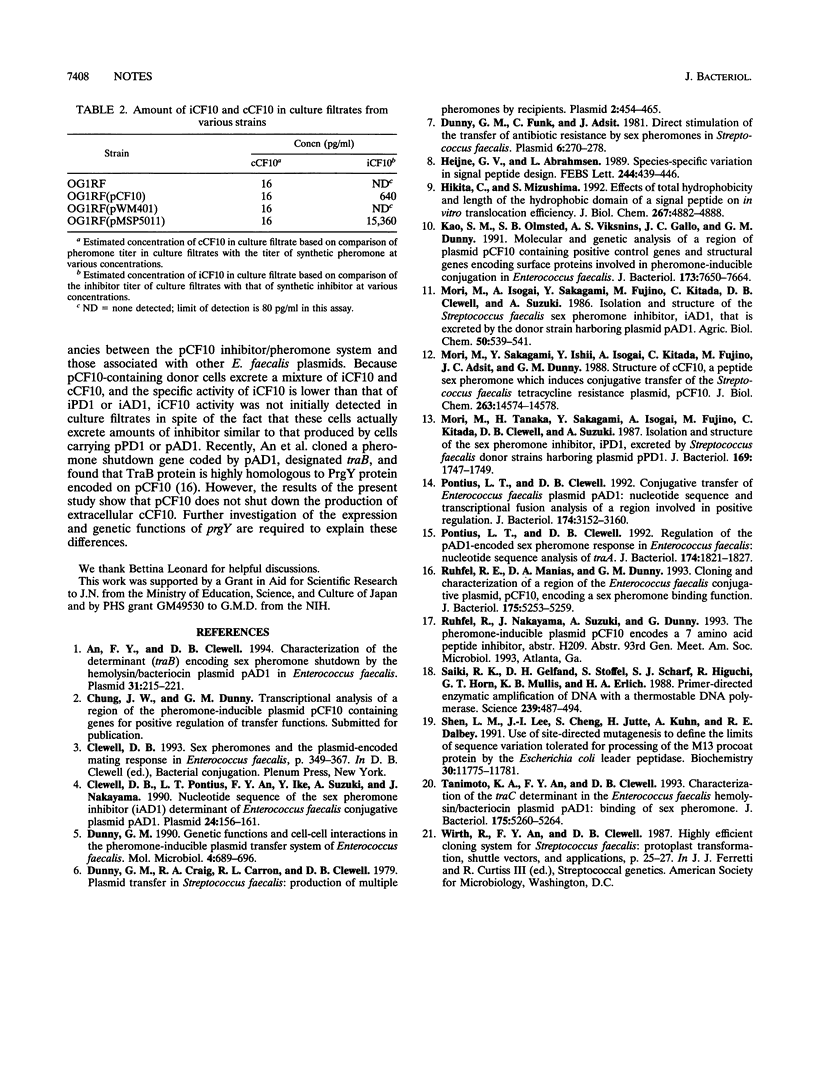
Conjugative transfer of the Enterococcus faecalis tetracycline resistance plasmid pCF10 is stimulated by a peptide pheromone, cCF10. Once a recipient strain acquires pCF10 and thus becomes a pheromone-responsive donor, cCF10 activity is no longer detected in culture filtrates. Here we show that pCF10 encodes a peptide inhibitor, iCF10, secreted by donor cells; this inhibitor antagonizes the cCF10 activity in culture filtrates. In order to detect and quantitate iCF10, we developed a reverse-phase high-performance liquid chromatography assay in which the inhibitor peptide elutes separately from the pheromone; this type of assay enabled us to determine that lack of pheromone activity in donor culture filtrates was due to secretion of a mixture of iCF10 and cCF10, rather than abolition of cCF10 secretion. The gene encoding iCF10, prgQ, is located on the EcoRI-C fragment of pCF10. The open reading frame comprising the prgQ gene encodes a 23-amino-acid precursor that resembles a signal peptide. This precursor is cleaved to the mature heptapeptide iCF10 during the secretion process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An F. Y., Clewell D. B. Characterization of the determinant (traB) encoding sex pheromone shutdown by the hemolysin/bacteriocin plasmid pAD1 in Enterococcus faecalis. Plasmid. 1994 Mar;31(2):215–221. doi: 10.1006/plas.1994.1023. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Pontius L. T., An F. Y., Ike Y., Suzuki A., Nakayama J. Nucleotide sequence of the sex pheromone inhibitor (iAD1) determinant of Enterococcus faecalis conjugative plasmid pAD1. Plasmid. 1990 Sep;24(2):156–161. doi: 10.1016/0147-619x(90)90019-9. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979 Jul;2(3):454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Dunny G. M. Genetic functions and cell-cell interactions in the pheromone-inducible plasmid transfer system of Enterococcus faecalis. Mol Microbiol. 1990 May;4(5):689–696. doi: 10.1111/j.1365-2958.1990.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Dunny G., Funk C., Adsit J. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid. 1981 Nov;6(3):270–278. doi: 10.1016/0147-619x(81)90035-4. [DOI] [PubMed] [Google Scholar]
- Hikita C., Mizushima S. Effects of total hydrophobicity and length of the hydrophobic domain of a signal peptide on in vitro translocation efficiency. J Biol Chem. 1992 Mar 5;267(7):4882–4888. [PubMed] [Google Scholar]
- Kao S. M., Olmsted S. B., Viksnins A. S., Gallo J. C., Dunny G. M. Molecular and genetic analysis of a region of plasmid pCF10 containing positive control genes and structural genes encoding surface proteins involved in pheromone-inducible conjugation in Enterococcus faecalis. J Bacteriol. 1991 Dec;173(23):7650–7664. doi: 10.1128/jb.173.23.7650-7664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Sakagami Y., Ishii Y., Isogai A., Kitada C., Fujino M., Adsit J. C., Dunny G. M., Suzuki A. Structure of cCF10, a peptide sex pheromone which induces conjugative transfer of the Streptococcus faecalis tetracycline resistance plasmid, pCF10. J Biol Chem. 1988 Oct 5;263(28):14574–14578. [PubMed] [Google Scholar]
- Mori M., Tanaka H., Sakagami Y., Isogai A., Fujino M., Kitada C., Clewell D. B., Suzuki A. Isolation and structure of the sex pheromone inhibitor, iPD1, excreted by Streptococcus faecalis donor strains harboring plasmid pPD1. J Bacteriol. 1987 Apr;169(4):1747–1749. doi: 10.1128/jb.169.4.1747-1749.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontius L. T., Clewell D. B. Conjugative transfer of Enterococcus faecalis plasmid pAD1: nucleotide sequence and transcriptional fusion analysis of a region involved in positive regulation. J Bacteriol. 1992 May;174(10):3152–3160. doi: 10.1128/jb.174.10.3152-3160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontius L. T., Clewell D. B. Regulation of the pAD1-encoded sex pheromone response in Enterococcus faecalis: nucleotide sequence analysis of traA. J Bacteriol. 1992 Mar;174(6):1821–1827. doi: 10.1128/jb.174.6.1821-1827.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhfel R. E., Manias D. A., Dunny G. M. Cloning and characterization of a region of the Enterococcus faecalis conjugative plasmid, pCF10, encoding a sex pheromone-binding function. J Bacteriol. 1993 Aug;175(16):5253–5259. doi: 10.1128/jb.175.16.5253-5259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shen L. M., Lee J. I., Cheng S. Y., Jutte H., Kuhn A., Dalbey R. E. Use of site-directed mutagenesis to define the limits of sequence variation tolerated for processing of the M13 procoat protein by the Escherichia coli leader peptidase. Biochemistry. 1991 Dec 24;30(51):11775–11781. doi: 10.1021/bi00115a006. [DOI] [PubMed] [Google Scholar]
- Tanimoto K., An F. Y., Clewell D. B. Characterization of the traC determinant of the Enterococcus faecalis hemolysin-bacteriocin plasmid pAD1: binding of sex pheromone. J Bacteriol. 1993 Aug;175(16):5260–5264. doi: 10.1128/jb.175.16.5260-5264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Abrahmsén L. Species-specific variation in signal peptide design. Implications for protein secretion in foreign hosts. FEBS Lett. 1989 Feb 27;244(2):439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]


