Abstract
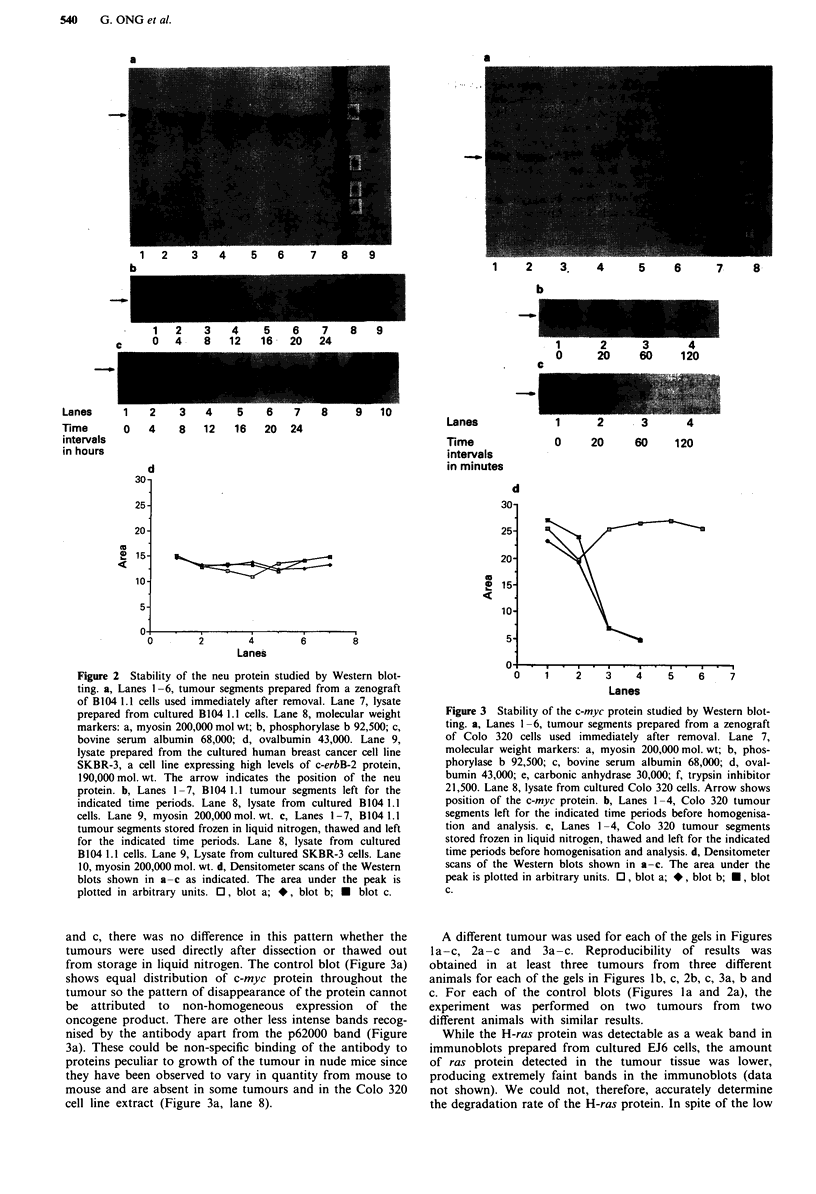
The means by which oncogenes and their products activate malignant transformation are currently under intense investigation. However, published papers on experiments using human tumour material do not always report in detail their methods of collection or storage of the specimens. In order to assess the stability of oncogene encoded proteins following collection or storage of human tumour biopsies, we have examined the rate of decay of the c-myc, neu and EGF-receptor proteins. Solid tumours, containing amplified copies of each oncogene, were established in nude mice and the stability of the oncogene protein in portions of each tumour, left in phosphate buffered saline at room temperature for varying time intervals, was examined by immunoblotting. Intact EGF-receptor and neu oncoproteins were present even after 24 h under these conditions while the c-myc protein was apparently rapidly degraded after 20 min. These data demonstrate that oncogene products decay at different rates after tumour resection and that collection of human biopsies should take this into account in order to provide the basis for consistent measurements of protein expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Aldrich T. H., Cordon-Cardo C. Expression of ras proto-oncogene proteins in normal human tissues. Oncogene. 1987 Mar;1(1):47–58. [PubMed] [Google Scholar]
- Gullick W. J., Berger M. S., Bennett P. L., Rothbard J. B., Waterfield M. D. Expression of the c-erbB-2 protein in normal and transformed cells. Int J Cancer. 1987 Aug 15;40(2):246–254. doi: 10.1002/ijc.2910400221. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Marsden J. J., Whittle N., Ward B., Bobrow L., Waterfield M. D. Expression of epidermal growth factor receptors on human cervical, ovarian, and vulval carcinomas. Cancer Res. 1986 Jan;46(1):285–292. [PubMed] [Google Scholar]
- Hudziak R. M., Lewis G. D., Winget M., Fendly B. M., Shepard H. M., Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989 Mar;9(3):1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris R. M., Lax I., Gullick W., Waterfield M. D., Ullrich A., Fridkin M., Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985 Mar;40(3):619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Maciewicz R. A., Knight P. J. Transmission densitometry of stained nitrocellulose paper. Anal Biochem. 1988 Nov 15;175(1):85–90. doi: 10.1016/0003-2697(88)90364-8. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Evan G. I., Bishop J. M. The protein encoded by the human proto-oncogene c-myc. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7742–7746. doi: 10.1073/pnas.81.24.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter A. L., Stern D. F., Vaidyanathan L., Decker S. J., Drebin J. A., Greene M. I., Weinberg R. A. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984 Dec 6;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Biology of the A-431 cell: a useful organism for hormone research. J Cell Biochem. 1983;23(1-4):191–202. doi: 10.1002/jcb.240230116. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984 Mar;98(3):1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]