Abstract
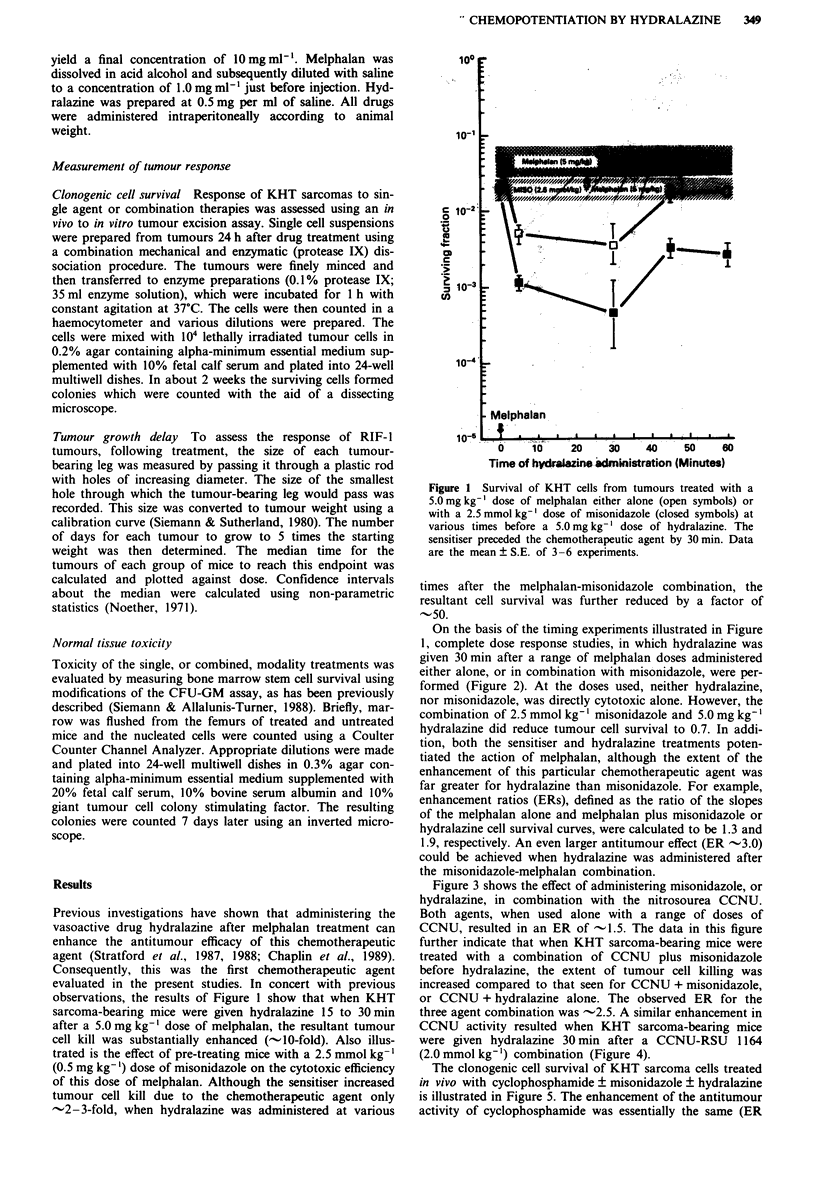
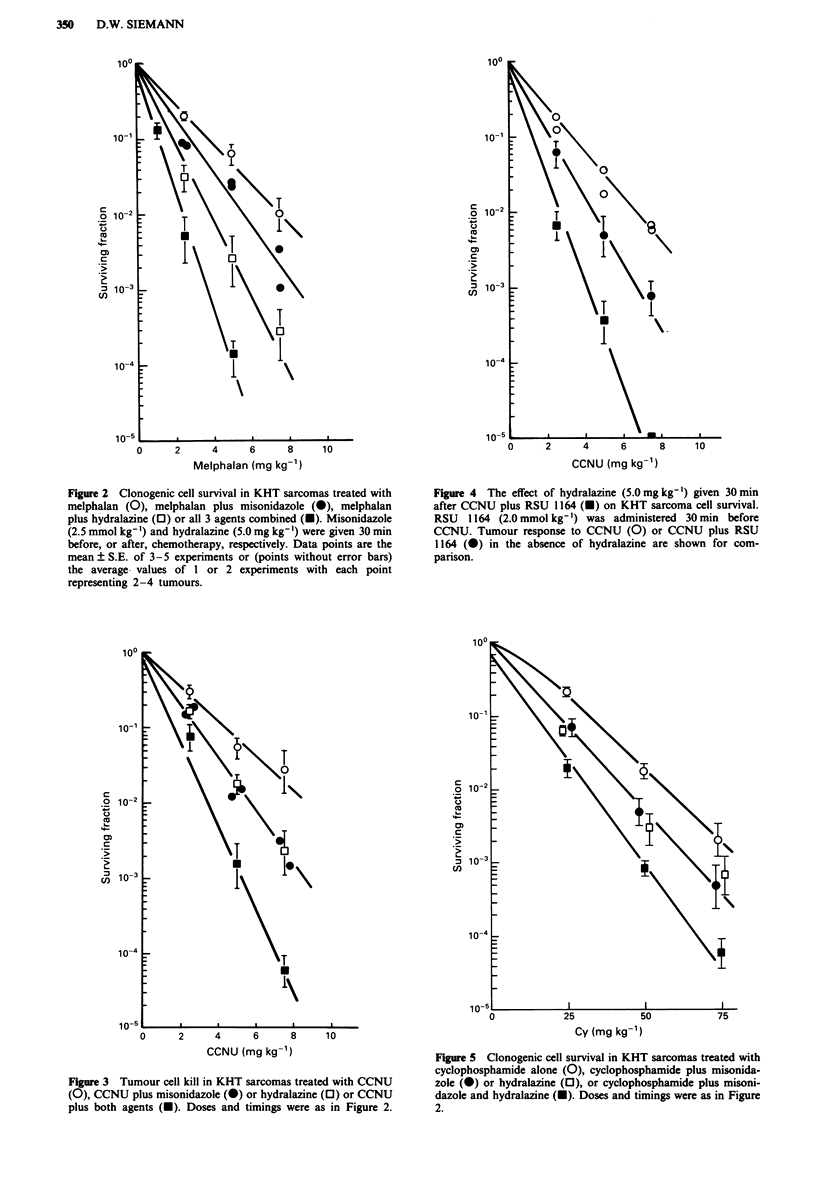
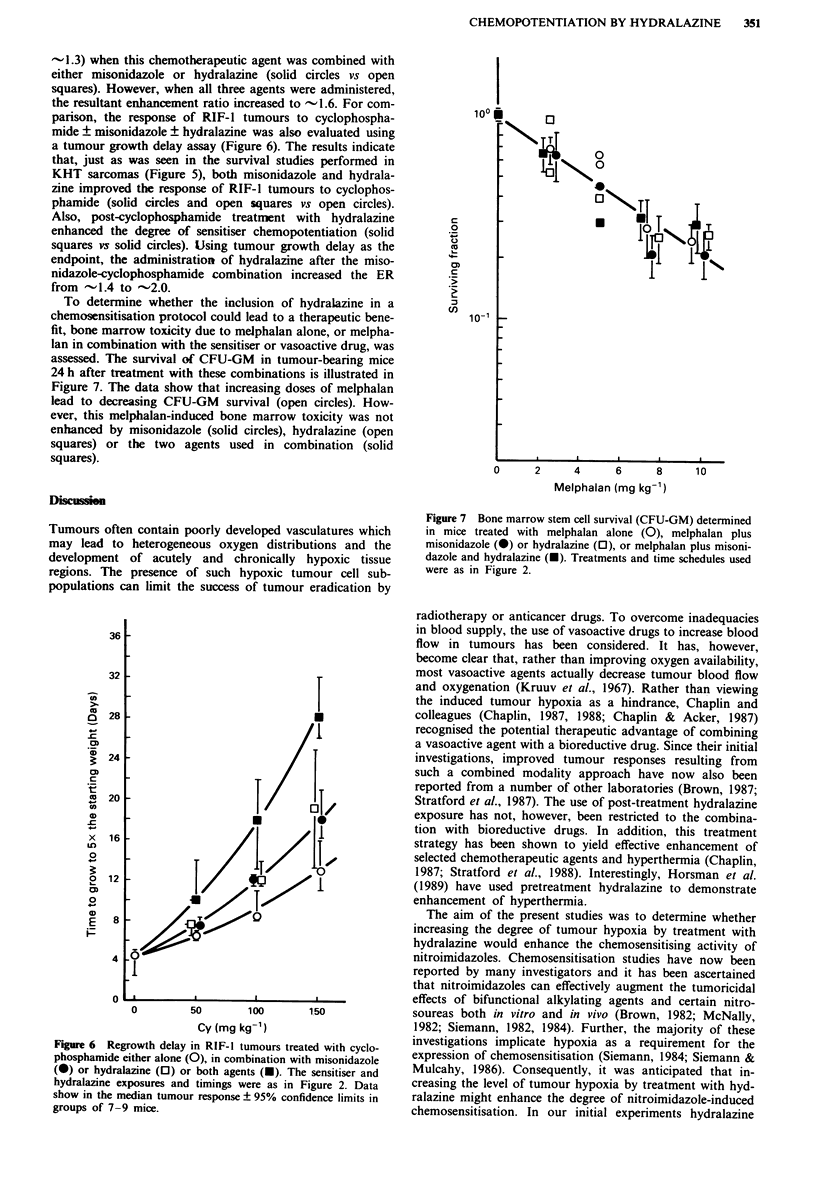
Nitroimidazoles have been shown to be potent sensitisers of certain clinically active chemotherapeutic agents. This process of chemopotentiation has been shown to be hypoxia-mediated. The present studies evaluated whether increasing the level of hypoxia in the tumour tissue, by treatment with the vasoactive agent hydralazine, could modify the chemosensitising ability of nitroheterocyclics. Administering either misonidazole or RSU 1164 before, or hydralazine after, the chemotherapeutic agents melphalan, cyclophosphamide or the nitrosourea CCNU, increased the extent of cell kill in both the KHT sarcoma and RIF-1 tumour. However, even greater enhancements could be achieved when hydralazine was used in treatment protocols in which a nitroimidazole was combined with chemotherapy. For example, a 5.0 mg kg-1 dose of hydralazine given 30 min after melphalan, or a 2.5 mmol kg-1 dose of misonidazole administered 30 min before melphalan, increased, compared to melphalan alone, the resultant tumour cell kill by factors of approximately 1.9 and approximately 1.3, respectively. By comparison, when hydralazine was given after the melphalan plus misonidazole combination, treatment efficacy was enhanced approximately 3-fold compared to melphalan alone. Yet in contrast to the results of the tumour response studies, the inclusion of hydralazine did not increase the bone marrow toxicity associated with the chemotherapeutic agent when used alone or in conjunction with a nitroimidazole. The results, therefore, imply that the addition of hydralazine to the chemotherapy, or chemotherapy-sensitiser protocol, led to a therapeutic advantage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Stratford I. J. Hypoxia-mediated nitro-heterocyclic drugs in the radio- and chemotherapy of cancer. An overview. Biochem Pharmacol. 1986 Jan 1;35(1):71–76. doi: 10.1016/0006-2952(86)90560-5. [DOI] [PubMed] [Google Scholar]
- Brown J. M. The mechanisms of cytotoxicity and chemosensitization by misonidazole and other nitroimidazoles. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):675–682. doi: 10.1016/0360-3016(82)90711-8. [DOI] [PubMed] [Google Scholar]
- Bush R. S., Jenkin R. D., Allt W. E., Beale F. A., Bean H., Dembo A. J., Pringle J. F. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl. 1978 Jun;3:302–306. [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. J., Acker B., Olive P. L. Potentiation of the tumor cytotoxicity of melphalan by vasodilating drugs. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1131–1135. doi: 10.1016/0360-3016(89)90267-8. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Acker B. The effect of hydralazine on the tumor cytotoxicity of the hypoxic cell cytotoxin RSU-1069: evidence for therapeutic gain. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):579–585. doi: 10.1016/0360-3016(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J. Postirradiation modification of tumor blood flow: a method to increase the effectiveness of chemical radiosensitizers. Radiat Res. 1988 Aug;115(2):292–302. [PubMed] [Google Scholar]
- Coleman C. N. Hypoxia in tumors: a paradigm for the approach to biochemical and physiologic heterogeneity. J Natl Cancer Inst. 1988 May 4;80(5):310–317. doi: 10.1093/jnci/80.5.310. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Christensen K. L., Overgaard J. Hydralazine-induced enhancement of hyperthermic damage in a C3H mammary carcinoma in vivo. Int J Hyperthermia. 1989 Mar-Apr;5(2):123–136. doi: 10.3109/02656738909140442. [DOI] [PubMed] [Google Scholar]
- Kallman R. F., Silini G., Van Putten L. M. Factors influencing the quantitative estimation of the in vivo survival of cells from solid tumors. J Natl Cancer Inst. 1967 Sep;39(3):539–549. [PubMed] [Google Scholar]
- Kennedy K. A., Rockwell S., Sartorelli A. C. Preferential activation of mitomycin C to cytotoxic metabolites by hypoxic tumor cells. Cancer Res. 1980 Jul;40(7):2356–2360. [PubMed] [Google Scholar]
- Kruuv J. A., Inch W. R., McCredie J. A. Blood flow and oxygenation of tumors in mice. II. Effects of vasodilator drugs. Cancer. 1967 Jan;20(1):60–65. doi: 10.1002/1097-0142(1967)20:1<60::aid-cncr2820200109>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- McNally N. J. Enhancement of chemotherapy agents. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):593–598. doi: 10.1016/0360-3016(82)90691-5. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Randhawa V. S. Misonidazole reduces blood flow in two experimental murine tumours. Br J Cancer. 1988 Aug;58(2):128–132. doi: 10.1038/bjc.1988.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Robinson B., Tannock I. F. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: potential implications for cell death in tumors. Cancer Res. 1986 Jun;46(6):2821–2826. [PubMed] [Google Scholar]
- Sartorelli A. C. The role of mitomycin antibiotics in the chemotherapy of solid tumors. Biochem Pharmacol. 1986 Jan 1;35(1):67–69. doi: 10.1016/0006-2952(86)90559-9. [DOI] [PubMed] [Google Scholar]
- Siemann D. W., Allalunis-Turner M. J. Potentiation of combination chemotherapy by nitroheterocyclics. Int J Radiat Oncol Biol Phys. 1988 Jul;15(1):129–134. doi: 10.1016/0360-3016(88)90356-2. [DOI] [PubMed] [Google Scholar]
- Siemann D. W. Modification of chemotherapy by nitroimidazoles. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1585–1594. doi: 10.1016/0360-3016(84)90508-x. [DOI] [PubMed] [Google Scholar]
- Siemann D. W. Potentiation of chemotherapy by hypoxic cell radiation sensitizers--a review. Int J Radiat Oncol Biol Phys. 1982 Jun;8(6):1029–1034. doi: 10.1016/0360-3016(82)90172-9. [DOI] [PubMed] [Google Scholar]
- Siemann D. W., Sutherland R. M. In vivo tumor response to single and multiple exposures of adriamycin. Eur J Cancer. 1980 Nov;16(11):1433–1440. doi: 10.1016/0014-2964(80)90052-3. [DOI] [PubMed] [Google Scholar]
- Stratford I. J., Adams G. E., Godden J., Nolan J., Howells N., Timpson N. Potentiation of the anti-tumour effect of melphalan by the vasoactive agent, hydralazine. Br J Cancer. 1988 Aug;58(2):122–127. doi: 10.1038/bjc.1988.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J. E., Rauth A. M. An in vitro assay to measure the viability of KHT tumor cells not previously exposed to culture conditions. Radiat Res. 1974 May;58(2):262–276. [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M., Lemmon M. J., Hirst V. K., Lee W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]


