Abstract
To obtain suitable cell lines for the immortalisation of human lymphocytes, we constructed a heteromyeloma between the murine myeloma Ag8 and human lymphocytes from a highly malignant polymorphic, centroblastic B-cell lymphoma. The thioguanine-resistant and HAT-sensitive heteromyeloma HAB-1 neither secretes nor contains cytoplasmatic immunoglobulins, the cells being EBV negative but positively stained for HLA-BC and the human proliferation marker Ki-67. The karyotype consists of about 50 murine and 20 human chromosomes. The HAB-1 cells grow in suspension and have a doubling rate of about 25-30 h. In fusion experiments with spleen cells from stomach carcinoma patients HAB-1 cells show a 5-7 times higher fusion efficiency than murine Ag8 cells or another heteromyeloma SPM4-0 and give stable antibody producing products. The cell line will be made available to interested scientists.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams P. G., Knost J. A., Clarke G., Wilburn S., Oldham R. K., Foon K. A. Determination of the optimal human cell lines for development of human hybridomas. J Immunol. 1983 Sep;131(3):1201–1204. [PubMed] [Google Scholar]
- Borrebaeck C. A., Danielsson L., Möller S. A. Human monoclonal antibodies produced from L-leucine methyl ester-treated and in vitro immunized peripheral blood lymphocytes. Biochem Biophys Res Commun. 1987 Nov 13;148(3):941–946. doi: 10.1016/s0006-291x(87)80223-1. [DOI] [PubMed] [Google Scholar]
- Carroll W. L., Thielemans K., Dilley J., Levy R. Mouse x human heterohybridomas as fusion partners with human B cell tumors. J Immunol Methods. 1986 May 1;89(1):61–72. doi: 10.1016/0022-1759(86)90032-3. [DOI] [PubMed] [Google Scholar]
- Cote R. J., Morrissey D. M., Houghton A. N., Beattie E. J., Jr, Oettgen H. F., Old L. J. Generation of human monoclonal antibodies reactive with cellular antigens. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2026–2030. doi: 10.1073/pnas.80.7.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Yamaguchi H., Oettgen H. F., Old L. J., Lloyd K. O. Analysis of the expression of N-glycolylneuraminic acid-containing gangliosides in cells and tissues using two human monoclonal antibodies. J Biol Chem. 1988 Dec 5;263(34):18507–18512. [PubMed] [Google Scholar]
- Gigliotti F., Smith L., Insel R. A. Reproducible production of protective human monoclonal antibodies by fusion of peripheral blood lymphocytes with a mouse myeloma cell line. J Infect Dis. 1984 Jan;149(1):43–47. doi: 10.1093/infdis/149.1.43. [DOI] [PubMed] [Google Scholar]
- Glassy M. C., Handley H. H., Hagiwara H., Royston I. UC 729-6, a human lymphoblastoid B-cell line useful for generating antibody-secreting human-human hybridomas. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6327–6331. doi: 10.1073/pnas.80.20.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunow R., Jahn S., Porstmann T., Kiessig S. S., Steinkellner H., Steindl F., Mattanovich D., Gürtler L., Deinhardt F., Katinger H. The high efficiency, human B cell immortalizing heteromyeloma CB-F7. Production of human monoclonal antibodies to human immunodeficiency virus. J Immunol Methods. 1988 Feb 10;106(2):257–265. doi: 10.1016/0022-1759(88)90206-2. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Roder J. C. Requirements for the establishment of high-titered human monoclonal antibodies against tetanus toxoid using the Epstein-Barr virus technique. J Immunol. 1981 Oct;127(4):1275–1280. [PubMed] [Google Scholar]
- Martin D., Larose Y., Hamel J., Lagacé J., Brodeur B. R. Heterohybridomas secreting human monoclonal antibodies against Haemophilus influenzae type b. Eur J Immunol. 1988 Apr;18(4):601–606. doi: 10.1002/eji.1830180417. [DOI] [PubMed] [Google Scholar]
- Olsson L., Kaplan H. S. Human-human hybridomas producing monoclonal antibodies of predefined antigenic specificity. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5429–5431. doi: 10.1073/pnas.77.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostberg L., Pursch E. Human X (mouse X human) hybridomas stably producing human antibodies. Hybridoma. 1983;2(4):361–367. doi: 10.1089/hyb.1983.2.361. [DOI] [PubMed] [Google Scholar]
- Ritts R. E., Jr, Ruiz-Argüelles A., Weyl K. G., Bradley A. L., Weihmeir B., Jacobsen D. J., Strehlo B. L. Establishment and characterization of a human non-secretory plasmacytoid cell line and its hybridization with human B cells. Int J Cancer. 1983 Feb 15;31(2):133–141. doi: 10.1002/ijc.2910310202. [DOI] [PubMed] [Google Scholar]
- Satoh J., Prabhakar B. S., Haspel M. V., Ginsberg-Fellner F., Notkins A. L. Human monoclonal autoantibodies that react with multiple endocrine organs. N Engl J Med. 1983 Jul 28;309(4):217–220. doi: 10.1056/NEJM198307283090405. [DOI] [PubMed] [Google Scholar]
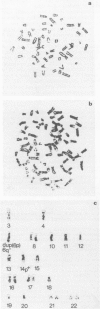
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Strike L. E., Devens B. H., Lundak R. L. Production of human-human hybridomas secreting antibody to sheep erythrocytes after in vitro immunization. J Immunol. 1984 Apr;132(4):1798–1803. [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N. N., Lam K. S., Calvo Riera F., Kaplan H. S. Construction and testing of mouse--human heteromyelomas for human monoclonal antibody production. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7308–7312. doi: 10.1073/pnas.80.23.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
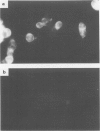
- Vollmers H. P., O'Connor R., Müller J., Kirchner T., Müller-Hermelink H. K. SC-1, a functional human monoclonal antibody against autologous stomach carcinoma cells. Cancer Res. 1989 May 1;49(9):2471–2476. [PubMed] [Google Scholar]
- Yamaura N., Makino M., Walsh L. J., Bruce A. W., Choe B. K. Production of monoclonal antibodies against prostatic acid phosphatase by in vitro immunization of human spleen cells. J Immunol Methods. 1985 Nov 28;84(1-2):105–116. doi: 10.1016/0022-1759(85)90419-3. [DOI] [PubMed] [Google Scholar]