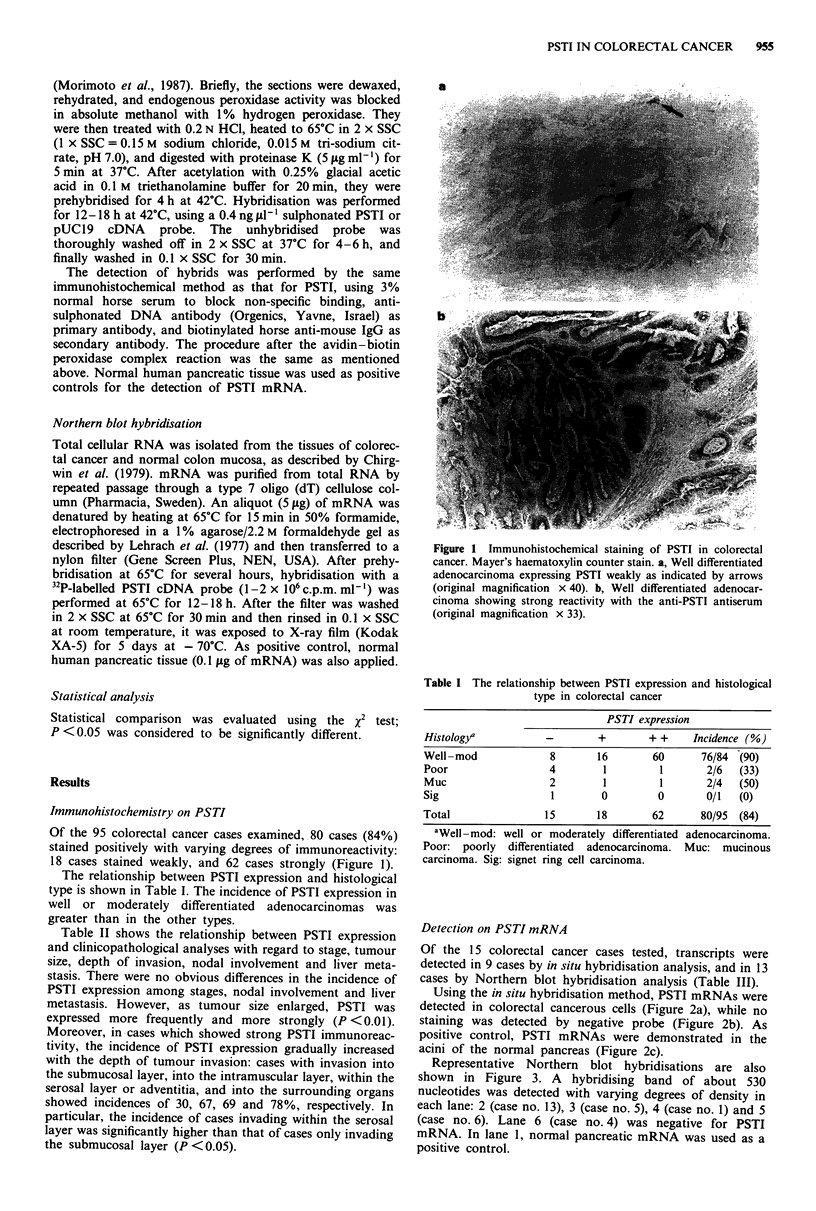
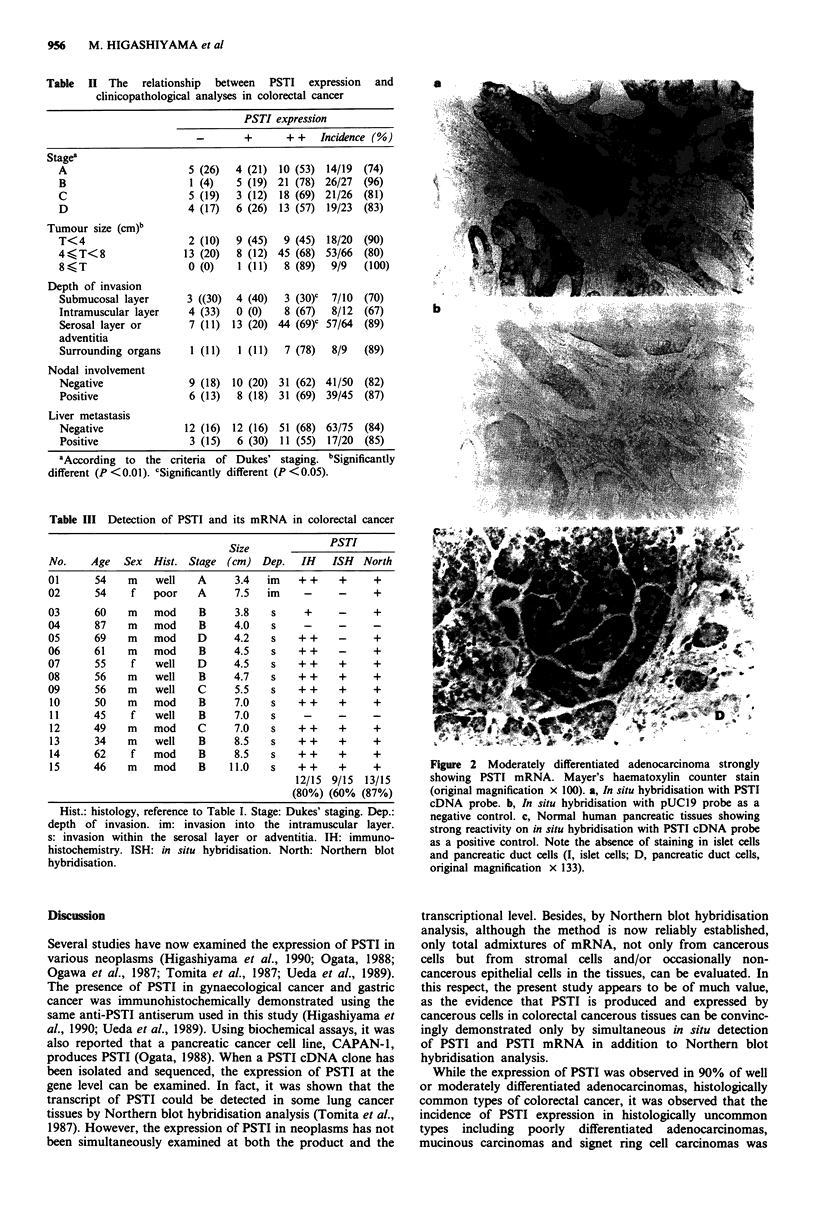
Abstract
We examined the expression of pancreatic secretory trypsin inhibitor (PSTI) in colorectal cancer by immunohistochemical staining using an anti-PSTI antiserum, an in situ hybridisation technique utilising sulphonated PSTI cDNA probe, and a Northern blot hybridisation method, using a 32P-labelled PSTI cDNA probe. Immunohistochemically, PSTI was detected in 80 of 95 (84%) colorectal cancer cases. Analyses with in situ hybridisation as well as Northern blot hybridisation demonstrated PSTI mRNAs in immunohistochemically positive cases, showing PSTI could be produced in colorectal cancerous cells. Histologically well or moderately differentiated adenocarcinoma showed higher incidence of PSTI immunoreactivity than the other types. Furthermore, the intensity of the immunohistochemical staining for PSTI increased the more cases advanced, particularly in regard to depth of invasion and tumour size. Thus, PSTI expression is widespread in colorectal cancer, and occurs more commonly in advanced cases. Considering the suggestion that PSTI is a growth-stimulating factor as an well as inhibitor to proteolytic proteinase, the present findings may indicate that PSTI expressed in colorectal cancerous cells may play a role possibly closely associated with tumour development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ades E. W., Hinson A., Chapuis-Cellier C., Arnaud P. Modulation of the immune response by plasma protease inhibitors. I. Alpha 2-macroglobulin and alpha 1-antitrypsin inhibit natural killing and antibody-dependent cell-mediated cytotoxicity. Scand J Immunol. 1982 Jan;15(1):109–113. doi: 10.1111/j.1365-3083.1982.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Bohe M., Borgström A., Lindström C., Ohlsson K. Pancreatic endoproteases and pancreatic secretory trypsin inhibitor immunoreactivity in human Paneth cells. J Clin Pathol. 1986 Jul;39(7):786–793. doi: 10.1136/jcp.39.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budowsky E. I., Sverdlov E. D., Monastyrskaya G. S. New method of selective and rapid modification of the cytidine residues. FEBS Lett. 1972 Sep 1;25(1):201–204. doi: 10.1016/0014-5793(72)80485-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fukayama M., Ogawa M., Hayashi Y., Koike M. Development of human pancreas. Immunohistochemical study of fetal pancreatic secretory proteins. Differentiation. 1986;31(2):127–133. doi: 10.1111/j.1432-0436.1986.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Fukuoka S., Fushiki T., Kitagawa Y., Sugimoto E., Iwai K. Growth stimulating activity on 3T3 fibroblasts of the molecular weight 6,500-peptide purified from rat pancreatic juice. Biochem Biophys Res Commun. 1986 Sep 14;139(2):545–550. doi: 10.1016/s0006-291x(86)80025-0. [DOI] [PubMed] [Google Scholar]
- Halila H., Huhtala M. L., Haglund C., Nordling S., Stenman U. H. Tumour-associated trypsin inhibitor (TATI) in human ovarian cyst fluid. Comparison with CA 125 and CEA. Br J Cancer. 1987 Aug;56(2):153–156. doi: 10.1038/bjc.1987.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hunt L. T., Barker W. C., Dayhoff M. O. Epidermal growth factor: internal duplication and probable relationship to pancreatic secretory trypsin inhibitor. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1020–1028. doi: 10.1016/0006-291x(74)90415-x. [DOI] [PubMed] [Google Scholar]
- Karashima S., Kataoka H., Itoh H., Maruyama R., Koono M. Prognostic significance of alpha-1-antitrypsin in early stage of colorectal carcinomas. Int J Cancer. 1990 Feb 15;45(2):244–250. doi: 10.1002/ijc.2910450207. [DOI] [PubMed] [Google Scholar]
- Kataoka H., Nabeshima K., Komada N., Koono M. New human colorectal carcinoma cell lines that secrete proteinase inhibitors in vitro. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(3):157–165. doi: 10.1007/BF02899077. [DOI] [PubMed] [Google Scholar]
- Kitahara T., Takatsuka Y., Fujimoto K. I., Tanaka S., Ogawa M., Kosaki G. Radioimmunoassay for human pancreatic secretory trypsin inhibitor: measurement of serum pancreatic secretory trypsin inhibitor in normal subjects and subjects with pancreatic diseases. Clin Chim Acta. 1980 Apr 25;103(2):135–143. doi: 10.1016/0009-8981(80)90205-3. [DOI] [PubMed] [Google Scholar]
- Kohga S., Harvey S. R., Weaver R. M., Markus G. Localization of plasminogen activators in human colon cancer by immunoperoxidase staining. Cancer Res. 1985 Apr;45(4):1787–1796. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., Sakagami Y., Hoshi H., McKeehan K. A. Two apparent human endothelial cell growth factors from human hepatoma cells are tumor-associated proteinase inhibitors. J Biol Chem. 1986 Apr 25;261(12):5378–5383. [PubMed] [Google Scholar]
- Morimoto H., Monden T., Shimano T., Higashiyama M., Tomita N., Murotani M., Matsuura N., Okuda H., Mori T. Use of sulfonated probes for in situ detection of amylase mRNA in formalin-fixed paraffin sections of human pancreas and submaxillary gland. Lab Invest. 1987 Dec;57(6):737–741. [PubMed] [Google Scholar]
- Niinobu T., Ogawa M., Shibata T., Murata A., Nishibe S., Mori T., Ogata N. Specific binding of human pancreatic secretory trypsin inhibitor to various cultured cells. Res Commun Chem Pathol Pharmacol. 1986 Aug;53(2):245–248. [PubMed] [Google Scholar]
- Ogata N. Demonstration of pancreatic secretory trypsin inhibitor in serum-free culture medium conditioned by the human pancreatic carcinoma cell line CAPAN-1. J Biol Chem. 1988 Sep 15;263(26):13427–13431. [PubMed] [Google Scholar]
- Ogawa M., Matsuura N., Higashiyama K., Mori T. Expression of pancreatic secretory trypsin inhibitor in various cancer cells. Res Commun Chem Pathol Pharmacol. 1987 Jan;55(1):137–140. [PubMed] [Google Scholar]
- Ogawa M., Tsushima T., Ohba Y., Ogawa N., Tanaka S., Ishida M., Mori T. Stimulation of DNA synthesis in human fibroblasts by human pancreatic secretory trypsin inhibitor. Res Commun Chem Pathol Pharmacol. 1985 Oct;50(1):155–158. [PubMed] [Google Scholar]
- Scheving L. A. Primary amino acid sequence similarity between human epidermal growth factor-urogastrone, human pancreatic secretory trypsin inhibitor, and members of porcine secretin family. Arch Biochem Biophys. 1983 Oct 15;226(2):411–413. doi: 10.1016/0003-9861(83)90309-0. [DOI] [PubMed] [Google Scholar]
- Tomita N., Horii A., Yamamoto T., Ogawa M., Mori T., Matsubara K. Expression of pancreatic secretory trypsin inhibitor gene in neoplastic tissues. FEBS Lett. 1987 Dec 10;225(1-2):113–119. doi: 10.1016/0014-5793(87)81141-9. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Höyhtyä M., Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987 Nov 25;907(3):191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Turpeinen U., Koivunen E., Stenman U. H. Reaction of a tumour-associated trypsin inhibitor with serine proteinases associated with coagulation and tumour invasion. Biochem J. 1988 Sep 15;254(3):911–914. doi: 10.1042/bj2540911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nakamura Y., Nishide J., Emi M., Ogawa M., Mori T., Matsubara K. Molecular cloning and nucleotide sequence of human pancreatic secretory trypsin inhibitor (PSTI) cDNA. Biochem Biophys Res Commun. 1985 Oct 30;132(2):605–612. doi: 10.1016/0006-291x(85)91176-3. [DOI] [PubMed] [Google Scholar]