Abstract
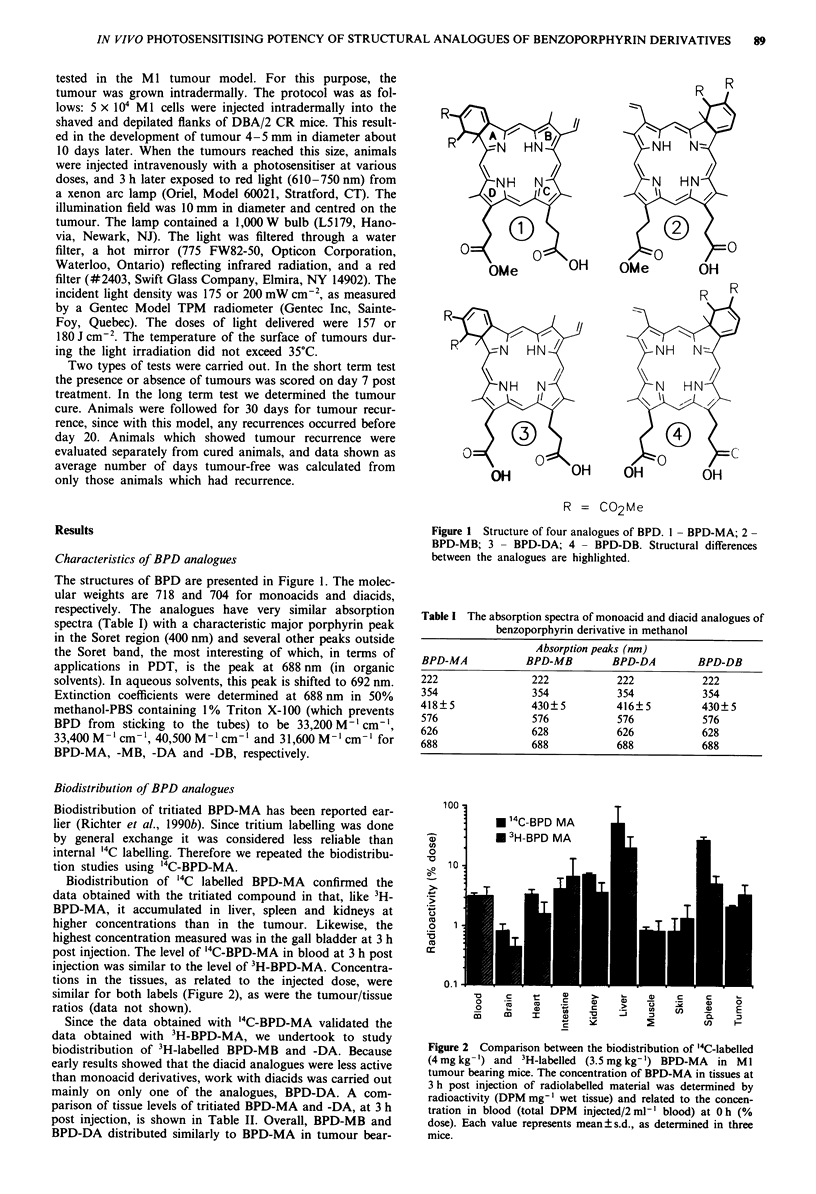
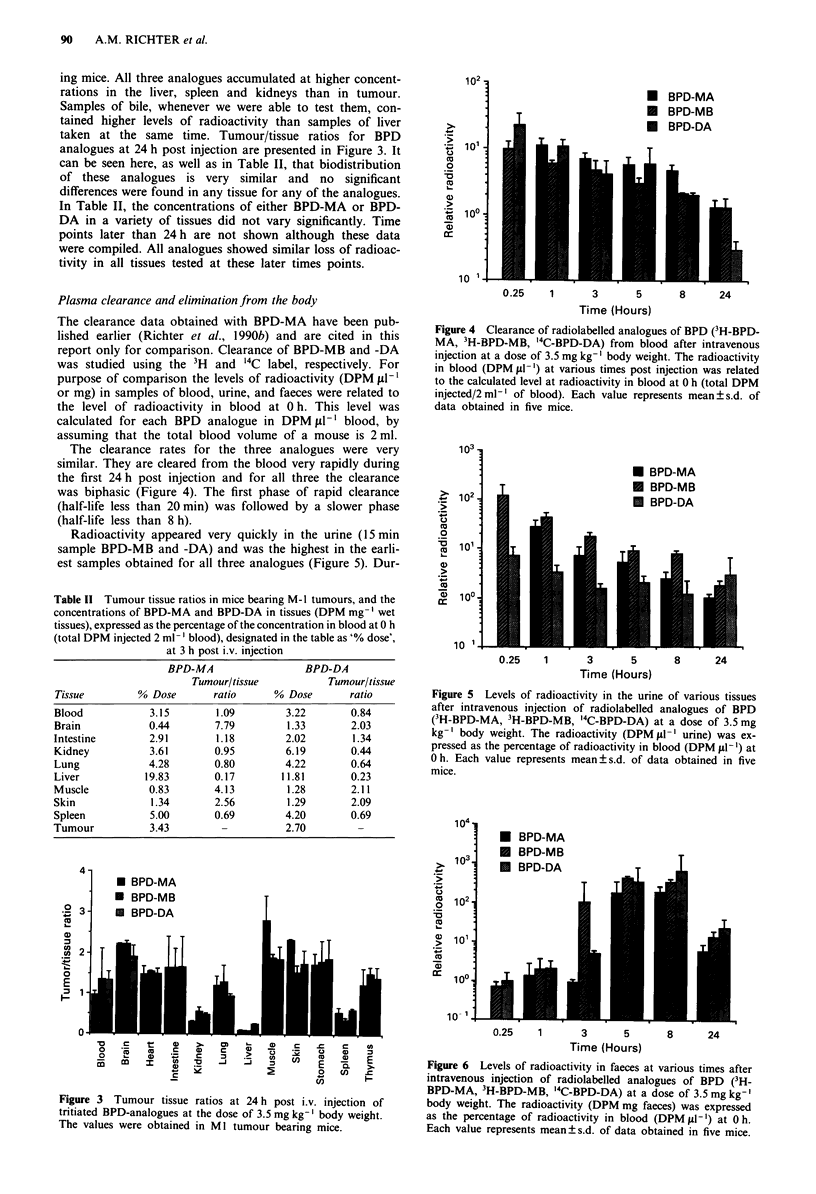
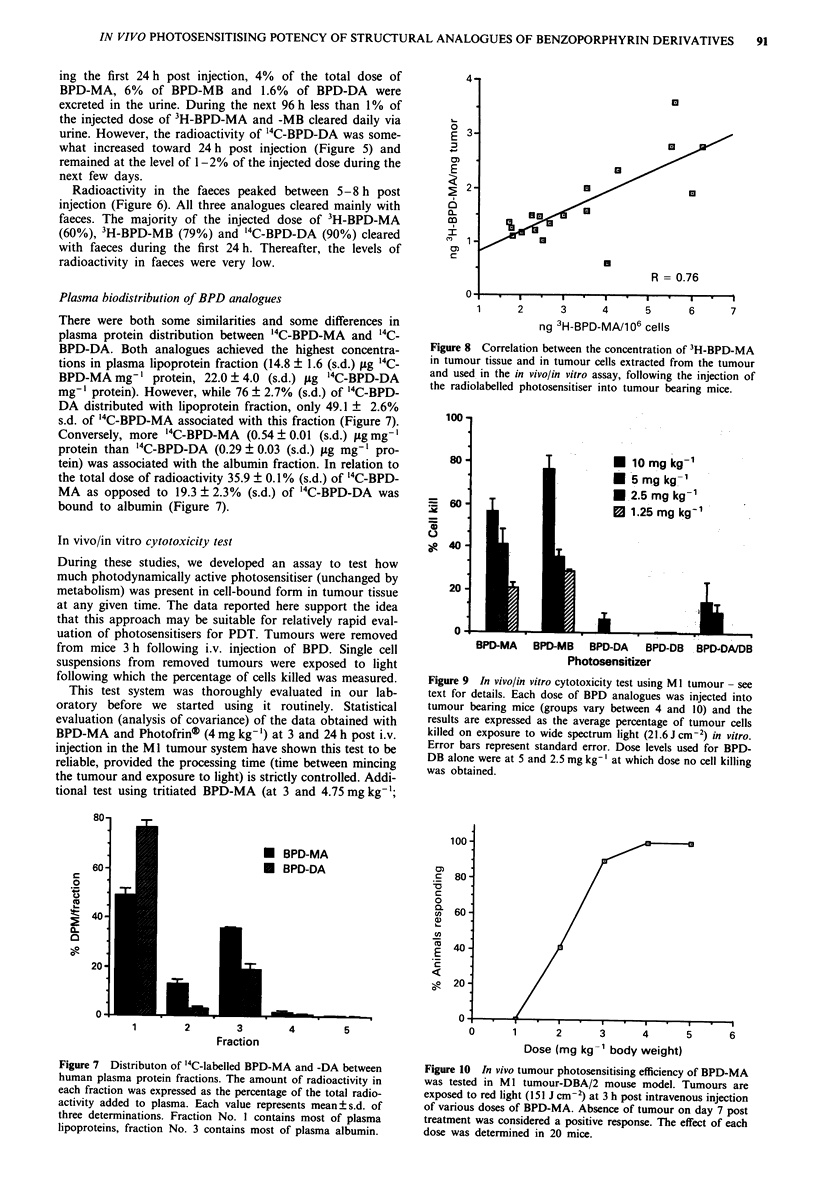
The in vivo characteristics of four analogues of benzoporphyrin derivative (BPD) have been investigated. Biodistribution data obtained in DBA/2J mice with BPD-MA (monoacid ring A analogue) which had been tritiated or internally labelled with 14C showed that both labelled materials acted in an essentially identical manner during the period of study. Biodistribution and clearance studies showed that relative distribution in a variety of mouse tissues was similar for all BPD analogues. M1 tumour cells (rhabdomyosarcoma in DBA/2J mice) taken from tumours excised from animals treated 3 h earlier with BPD, and tested in vitro for photosensitivity provided evidence that significant levels of photosensitiser detected in tumour was both active and associated with tumour cells. The monoacid forms of BPD were found to be much more photodynamically active in this test than were the diacid analogues. The ability of the analogues to ablate tumours in mice by photodynamic therapy was also tested. Again, BPD-MA and BPD-MB proved to be measurably better than the diacid analogues. These findings are discussed in reference to structural and physical differences between the analogues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison B. A., Pritchard P. H., Richter A. M., Levy J. G. The plasma distribution of benzoporphyrin derivative and the effects of plasma lipoproteins on its biodistribution. Photochem Photobiol. 1990 Sep;52(3):501–507. doi: 10.1111/j.1751-1097.1990.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Bellnier D. A., Ho Y. K., Pandey R. K., Missert J. R., Dougherty T. J. Distribution and elimination of Photofrin II in mice. Photochem Photobiol. 1989 Aug;50(2):221–228. doi: 10.1111/j.1751-1097.1989.tb04152.x. [DOI] [PubMed] [Google Scholar]
- Brasseur N., Ali H., Langlois R., van Lier J. E. Biological activities of phthalocyanines--IX. Photosensitization of V-79 Chinese hamster cells and EMT-6 mouse mammary tumor by selectively sulfonated zinc phthalocyanines. Photochem Photobiol. 1988 May;47(5):705–711. doi: 10.1111/j.1751-1097.1988.tb02768.x. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Studies on the structure of porphyrins contained in Photofrin II. Photochem Photobiol. 1987 Nov;46(5):569–573. doi: 10.1111/j.1751-1097.1987.tb04815.x. [DOI] [PubMed] [Google Scholar]
- Jori G., Beltramini M., Reddi E., Salvato B., Pagnan A., Ziron L., Tomio L., Tsanov T. Evidence for a major role of plasma lipoproteins as hematoporphyrin carriers in vivo. Cancer Lett. 1984 Oct;24(3):291–297. doi: 10.1016/0304-3835(84)90025-9. [DOI] [PubMed] [Google Scholar]
- Kessel D., Thompson P., Musselman B., Chang C. K. Chemistry of hematoporphyrin-derived photosensitizers. Photochem Photobiol. 1987 Nov;46(5):563–568. doi: 10.1111/j.1751-1097.1987.tb04814.x. [DOI] [PubMed] [Google Scholar]
- Kreimer-Birnbaum M. Modified porphyrins, chlorins, phthalocyanines, and purpurins: second-generation photosensitizers for photodynamic therapy. Semin Hematol. 1989 Apr;26(2):157–173. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Richter A. M., Cerruti-Sola S., Sternberg E. D., Dolphin D., Levy J. G. Biodistribution of tritiated benzoporphyrin derivative (3H-BPD-MA), a new potent photosensitizer, in normal and tumor-bearing mice. J Photochem Photobiol B. 1990 Apr 15;5(2):231–244. doi: 10.1016/1011-1344(90)80008-l. [DOI] [PubMed] [Google Scholar]
- Richter A. M., Kelly B., Chow J., Liu D. J., Towers G. H., Dolphin D., Levy J. G. Preliminary studies on a more effective phototoxic agent than hematoporphyrin. J Natl Cancer Inst. 1987 Dec;79(6):1327–1332. [PubMed] [Google Scholar]
- Richter A. M., Waterfield E., Jain A. K., Sternberg E. D., Dolphin D., Levy J. G. In vitro evaluation of phototoxic properties of four structurally related benzoporphyrin derivatives. Photochem Photobiol. 1990 Sep;52(3):495–500. doi: 10.1111/j.1751-1097.1990.tb01791.x. [DOI] [PubMed] [Google Scholar]
- Rotenberg M., Cohen S., Margalit R. Thermodynamics of porphyrin binding to serum albumin: effects of temperature, of porphyrin species and of albumin-carried fatty acids. Photochem Photobiol. 1987 Nov;46(5):689–693. doi: 10.1111/j.1751-1097.1987.tb04833.x. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Lee J. A., Morris M. D., Felts J. M. Characterization of plasma lipoproteins separated and purified by agarose-column chromatography. Biochem J. 1974 Apr;139(1):89–95. doi: 10.1042/bj1390089. [DOI] [PMC free article] [PubMed] [Google Scholar]


