Abstract
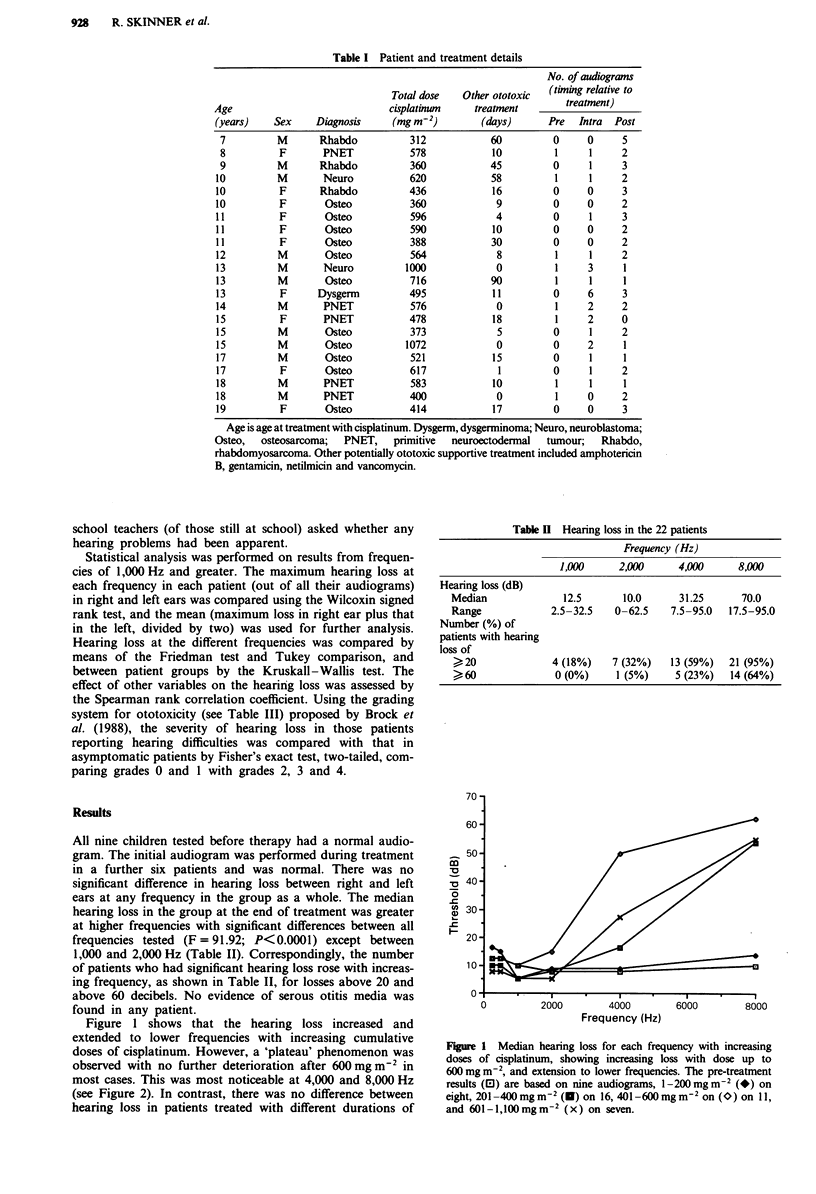
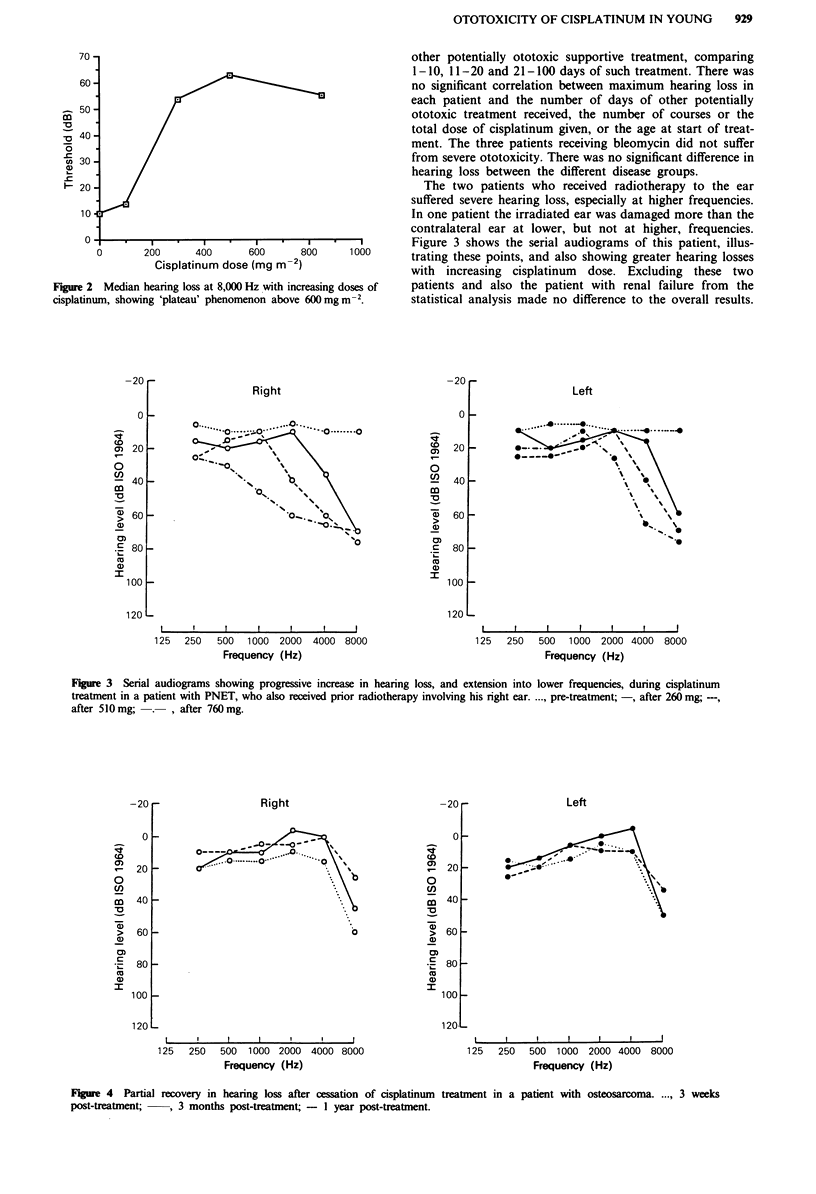
Twenty-two children and adolescents who had received cisplatinum for the treatment of solid tumours underwent audiometry to ascertain the extent of hearing damage. Five patients complained of hearing difficulties, causing difficulty at school in one child. Hearing loss greater than 20 decibels occurred in four patients at 1,000 Hz, seven at 2,000 Hz, 13 at 4,000 Hz and 21 at 8,000 Hz. Median hearing loss was greater at higher frequencies (P less than 0.0001), and with increasing cumulative dose of cisplatinum. However, a 'plateau' phenomenon was observed, with no apparent further deterioration in hearing loss at doses greater than 600 mg m-2. Two children who had received prior aural radiotherapy had severe hearing loss. Severe, mostly asymptomatic, ototoxicity is common in children given cisplatinum. However, there is considerable interpatient variability in the hearing loss suffered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Markulis N. V., Beckley S., Priore R., Mettlin C. Auditory toxicity effects of long-term cis-dichlorodiammineplatinum II therapy in genitourinary cancer patients. J Surg Oncol. 1981;16(2):111–123. doi: 10.1002/jso.2930160203. [DOI] [PubMed] [Google Scholar]
- Brock P., Pritchard J., Bellman S., Pinkerton C. R. Ototoxicity of high-dose cis-platinum in children. Med Pediatr Oncol. 1988;16(5):368–369. doi: 10.1002/mpo.2950160517. [DOI] [PubMed] [Google Scholar]
- Ettinger L. J., Douglass H. O., Jr, Higby D. J., Mindell E. R., Nime F., Ghoorah J., Freeman A. I. Adjuvant adriamycin and cis-diamminedichloroplatinum (cis-platinum) in primary osteosarcoma. Cancer. 1981 Jan 15;47(2):248–254. doi: 10.1002/1097-0142(19810115)47:2<248::aid-cncr2820470208>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kopelman J., Budnick A. S., Sessions R. B., Kramer M. B., Wong G. Y. Ototoxicity of high-dose cisplatin by bolus administration in patients with advanced cancers and normal hearing. Laryngoscope. 1988 Aug;98(8 Pt 1):858–864. doi: 10.1288/00005537-198808000-00014. [DOI] [PubMed] [Google Scholar]
- Mahoney D. H., Jr, Weaver T., Steuber C. P., Starling K. A. Ototoxicity with cisplatin therapy. J Pediatr. 1983 Dec;103(6):1006–1007. doi: 10.1016/s0022-3476(83)80747-1. [DOI] [PubMed] [Google Scholar]
- McHaney V. A., Thibadoux G., Hayes F. A., Green A. A. Hearing loss in children receiving cisplatin chemotherapy. J Pediatr. 1983 Feb;102(2):314–317. doi: 10.1016/s0022-3476(83)80551-4. [DOI] [PubMed] [Google Scholar]
- Melamed L. B., Selim M. A., Schuchman D. Cisplatin ototoxicity in gynecologic cancer patients. A preliminary report. Cancer. 1985 Jan 1;55(1):41–43. doi: 10.1002/1097-0142(19850101)55:1<41::aid-cncr2820550106>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Moroso M. J., Blair R. L. A review of cis-platinum ototoxicity. J Otolaryngol. 1983 Dec;12(6):365–369. [PubMed] [Google Scholar]
- Ozols R. F., Deisseroth A. B., Javadpour N., Barlock A., Messerschmidt G. L., Young R. C. Treatment of poor prognosis nonseminomatous testicular cancer with a "high-dose" platinum combination chemotherapy regimen. Cancer. 1983 May 15;51(10):1803–1807. doi: 10.1002/1097-0142(19830515)51:10<1803::aid-cncr2820511008>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Pinkerton C. R., Pritchard J., Spitz L. High complete response rate in children with advanced germ cell tumors using cisplatin-containing combination chemotherapy. J Clin Oncol. 1986 Feb;4(2):194–199. doi: 10.1200/JCO.1986.4.2.194. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Kefford R. F., Grant J. M., Coates A. S., Fox R. M., Tattersall M. H. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treat Rep. 1982 Jan;66(1):19–23. [PubMed] [Google Scholar]
- Ruiz L., Gilden J., Jaffe N., Robertson R., Wang Y. M. Auditory function in pediatric osteosarcoma patients treated with multiple doses of cis-diamminedichloroplatinum(II). Cancer Res. 1989 Feb 1;49(3):742–744. [PubMed] [Google Scholar]
- Salem P. A., Jabboury K. W., Khalil M. F. Severe nephrotoxicity: a probable complication of cis-dichlorodiammineplatinum (II) and cephalothin-gentamicin therapy. Oncology. 1982;39(1):31–32. doi: 10.1159/000225600. [DOI] [PubMed] [Google Scholar]
- Stadnicki S. W., Fleischman R. W., Schaeppi U., Merriam P. Cis-dichlorodiammineplatinum (II) (NSC-119875): hearing loss and other toxic effects in rhesus monkeys. Cancer Chemother Rep. 1975 May-Jun;59(3):467–480. [PubMed] [Google Scholar]
- Strauss M., Towfighi J., Lord S., Lipton A., Harvey H. A., Brown B. Cis-platinum ototoxicity: clinical experience and temporal bone histopathology. Laryngoscope. 1983 Dec;93(12):1554–1559. doi: 10.1288/00005537-198312000-00007. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Pillow J., Waters K. D., Keir E. Enhanced cis-platinum ototoxicity in children with brain tumours who have received simultaneous or prior cranial irradiation. Med Pediatr Oncol. 1989;17(1):48–52. doi: 10.1002/mpo.2950170110. [DOI] [PubMed] [Google Scholar]


