Abstract
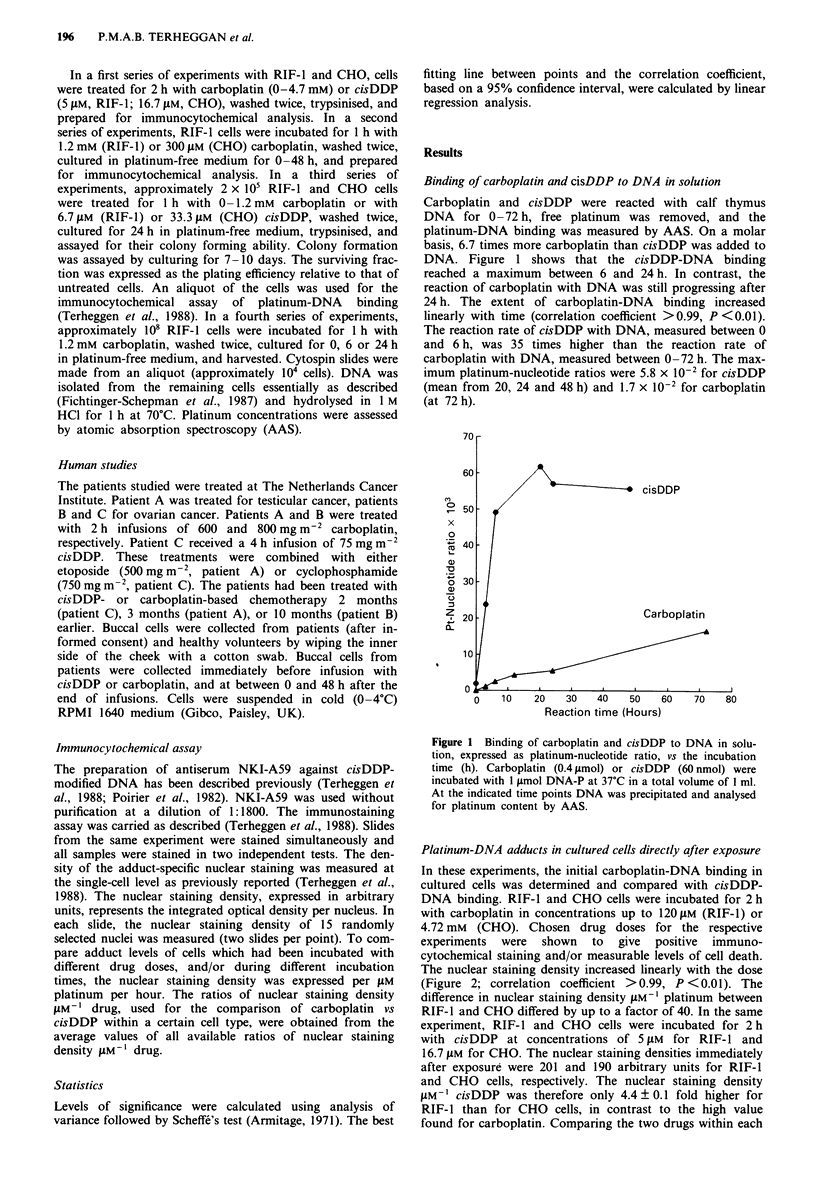
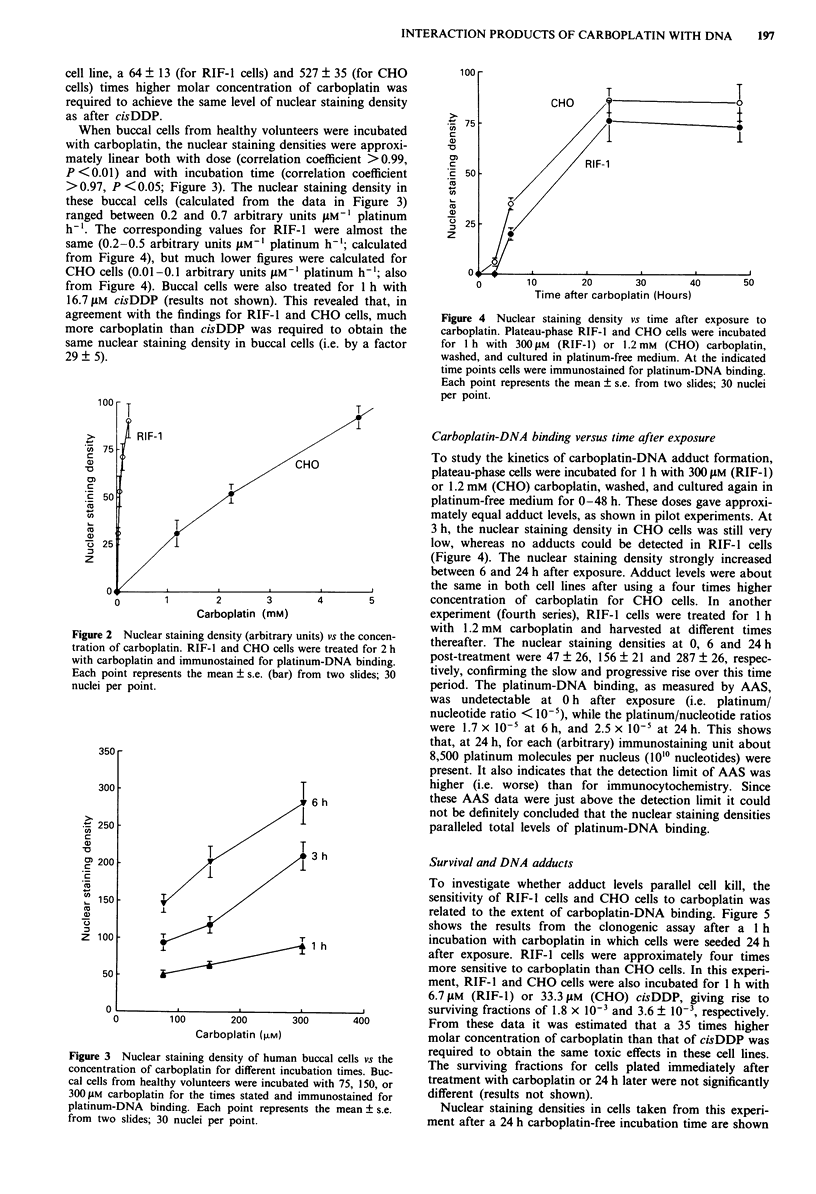
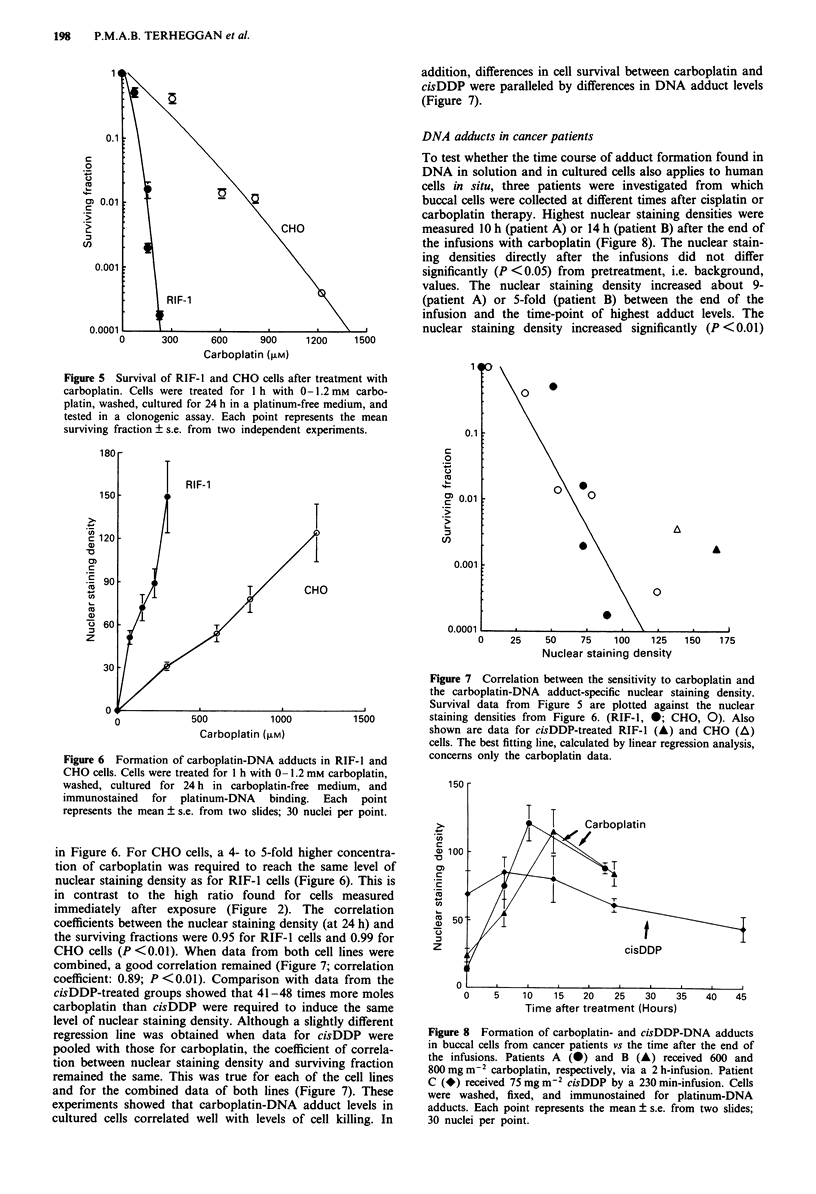
Binding of the cytostatic drug carboplatin to DNA was studied in solution, in RIF-1 and CHO cell lines and in human buccal cells after in vitro or in situ drug exposure. Results were compared with DNA adduction by cisplatin. The rate of binding in solution, determined by atomic absorption spectroscopy, was 35 times lower for carboplatin than for cisplatin. Adduct formation in cells in vitro was determined in a quantitative immunostaining assay. Staining intensities after carboplatin treatment were at least 29 times lower than after an equimolar dose of cisplatin. For RIF-1 and CHO cells, maximum levels of carboplatin-induced DNA modification were obtained 24 h after treatment; these levels correlated with cell killing. Adduct-specific staining in buccal cells from two carboplatin-treated patients increased 5-7 fold between 0 and 14 h after infusion, reaching a maximum at 10-14 h. This strongly contrasts with buccal cells from a cisplatin-treated patient, in which the adduct-specific staining signal increased by only 23% between 0 and 6 h after infusion, and then declined. This difference in the rate of adduct formation in vivo is consistent with the in vitro data.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedford P., Shellard S. A., Walker M. C., Whelan R. D., Masters J. R., Hill B. T. Differential expression of collateral sensitivity or resistance to cisplatin in human bladder carcinoma cell lines pre-exposed in vitro to either X-irradiation or cisplatin. Int J Cancer. 1987 Nov 15;40(5):681–686. doi: 10.1002/ijc.2910400519. [DOI] [PubMed] [Google Scholar]
- Calvert A. H., Harland S. J., Newell D. R., Siddik Z. H., Jones A. C., McElwain T. J., Raju S., Wiltshaw E., Smith I. E., Baker J. M. Early clinical studies with cis-diammine-1,1-cyclobutane dicarboxylate platinum II. Cancer Chemother Pharmacol. 1982;9(3):140–147. doi: 10.1007/BF00257742. [DOI] [PubMed] [Google Scholar]
- Evans B. D., Raju K. S., Calvert A. H., Harland S. J., Wiltshaw E. Phase II study of JM8, a new platinum analog, in advanced ovarian carcinoma. Cancer Treat Rep. 1983 Nov;67(11):997–1000. [PubMed] [Google Scholar]
- Knox R. J., Friedlos F., Lydall D. A., Roberts J. J. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986 Apr;46(4 Pt 2):1972–1979. [PubMed] [Google Scholar]
- Kuppen P. J., Schuitemaker H., van 't Veer L. J., de Bruijn E. A., van Oosterom A. T., Schrier P. I. cis-diamminedichloroplatinum(II)-resistant sublines derived from two human ovarian tumor cell lines. Cancer Res. 1988 Jun 15;48(12):3355–3359. [PubMed] [Google Scholar]
- Lippard S. J. New chemistry of an old molecule: cis-[Pt(NH3)2Cl2]. Science. 1982 Dec 10;218(4577):1075–1082. doi: 10.1126/science.6890712. [DOI] [PubMed] [Google Scholar]
- Poirier M. C., Lippard S. J., Zwelling L. A., Ushay H. M., Kerrigan D., Thill C. C., Santella R. M., Grunberger D., Yuspa S. H. Antibodies elicited against cis-diamminedichloroplatinum(II)-modified DNA are specific for cis-diamminedichloroplatinum(II)-DNA adducts formed in vivo and in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6443–6447. doi: 10.1073/pnas.79.21.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed E., Ozols R. F., Tarone R., Yuspa S. H., Poirier M. C. Platinum-DNA adducts in leukocyte DNA correlate with disease response in ovarian cancer patients receiving platinum-based chemotherapy. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5024–5028. doi: 10.1073/pnas.84.14.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Friedlos F. Quantitative estimation of cisplatin-induced DNA interstrand cross-links and their repair in mammalian cells: relationship to toxicity. Pharmacol Ther. 1987;34(2):215–246. doi: 10.1016/0163-7258(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg B. Fundamental studies with cisplatin. Cancer. 1985 May 15;55(10):2303–23l6. doi: 10.1002/1097-0142(19850515)55:10<2303::aid-cncr2820551002>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Siddik Z. H., Jones M., Boxall F. E., Harrap K. R. Comparative distribution and excretion of carboplatin and cisplatin in mice. Cancer Chemother Pharmacol. 1988;21(1):19–24. doi: 10.1007/BF00262732. [DOI] [PubMed] [Google Scholar]
- Siddik Z. H., Newell D. R., Boxall F. E., Harrap K. R. The comparative pharmacokinetics of carboplatin and cisplatin in mice and rats. Biochem Pharmacol. 1987 Jun 15;36(12):1925–1932. doi: 10.1016/0006-2952(87)90490-4. [DOI] [PubMed] [Google Scholar]
- Terheggen P. M., Dijkman R., Begg A. C., Dubbelman R., Floot B. G., Hart A. A., den Engelse L. Monitoring of interaction products of cis-diamminedichloroplatinum(II) and cis-diammine(1,1-cyclobutanedicarboxylato)platinum(II) with DNA in cells from platinum-treated cancer patients. Cancer Res. 1988 Oct 1;48(19):5597–5603. [PubMed] [Google Scholar]
- Terheggen P. M., Emondt J. Y., Floot B. G., Dijkman R., Schrier P. I., den Engelse L., Begg A. C. Correlation between cell killing by cis-diamminedichloroplatinum(II) in six mammalian cell lines and binding of a cis-diamminedichloroplatinum(II)-DNA antiserum. Cancer Res. 1990 Jun 15;50(12):3556–3561. [PubMed] [Google Scholar]
- Terheggen P. M., van der Hoop R. G., Floot B. G., Gispen W. H. Cellular distribution of cis-diamminedichloroplatinum(II)-DNA binding in rat dorsal root spinal ganglia: effect of the neuroprotecting peptide ORG.2766. Toxicol Appl Pharmacol. 1989 Jun 15;99(2):334–343. doi: 10.1016/0041-008x(89)90015-x. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Zwelling L. A., Kohn K. W. Mechanism of action of cis-dichlorodiammineplatinum(II). Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1439–1444. [PubMed] [Google Scholar]


