Abstract
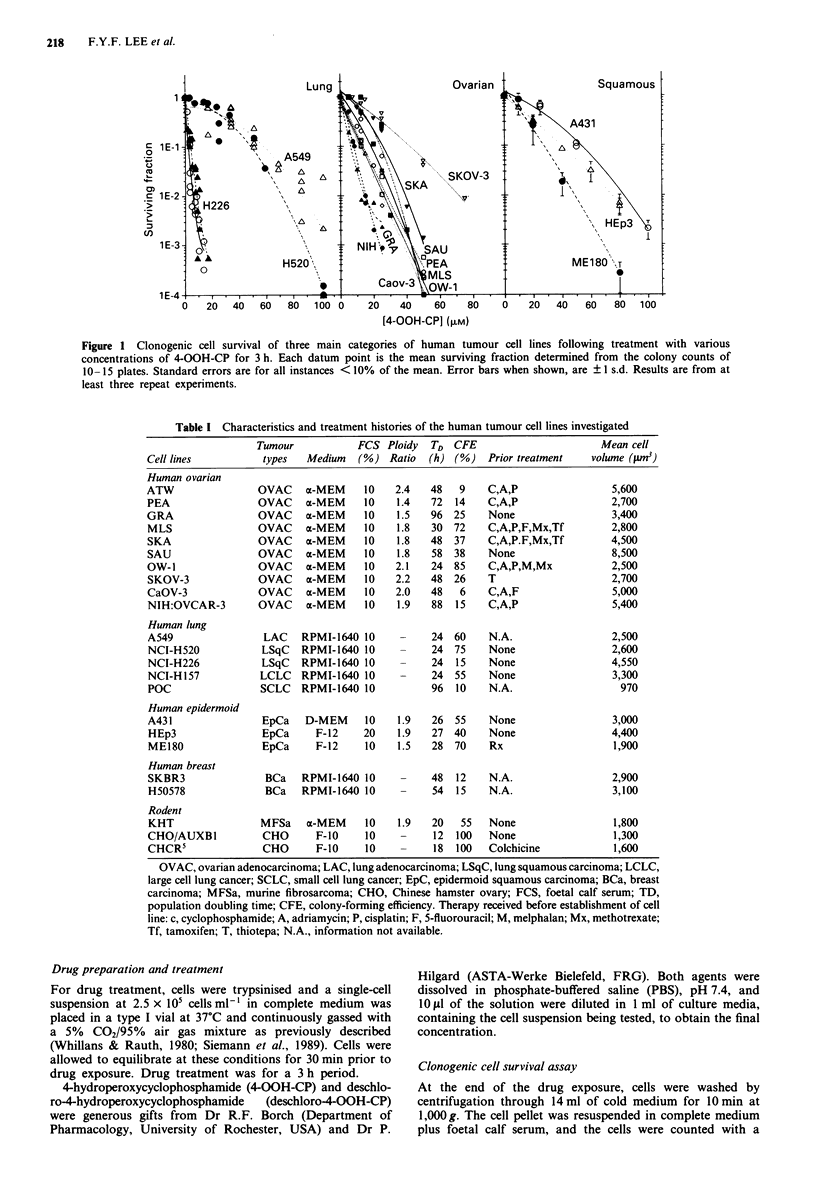
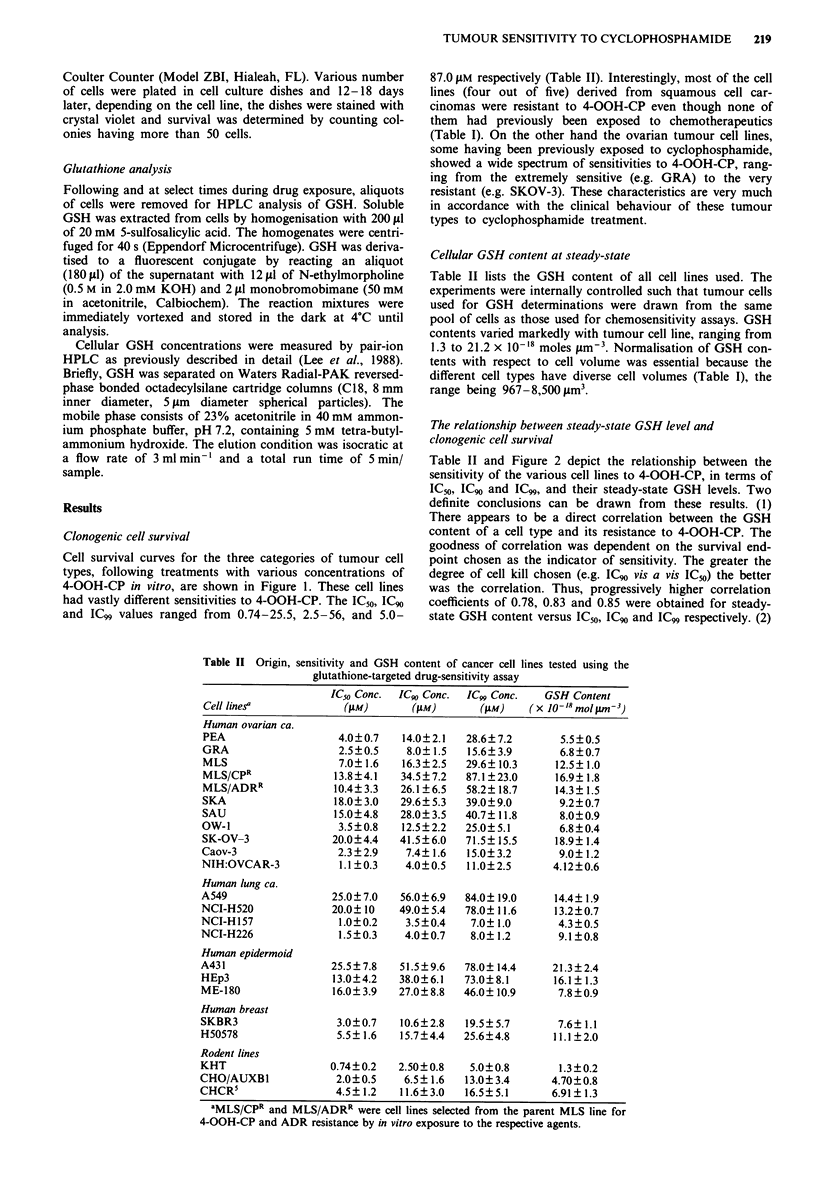
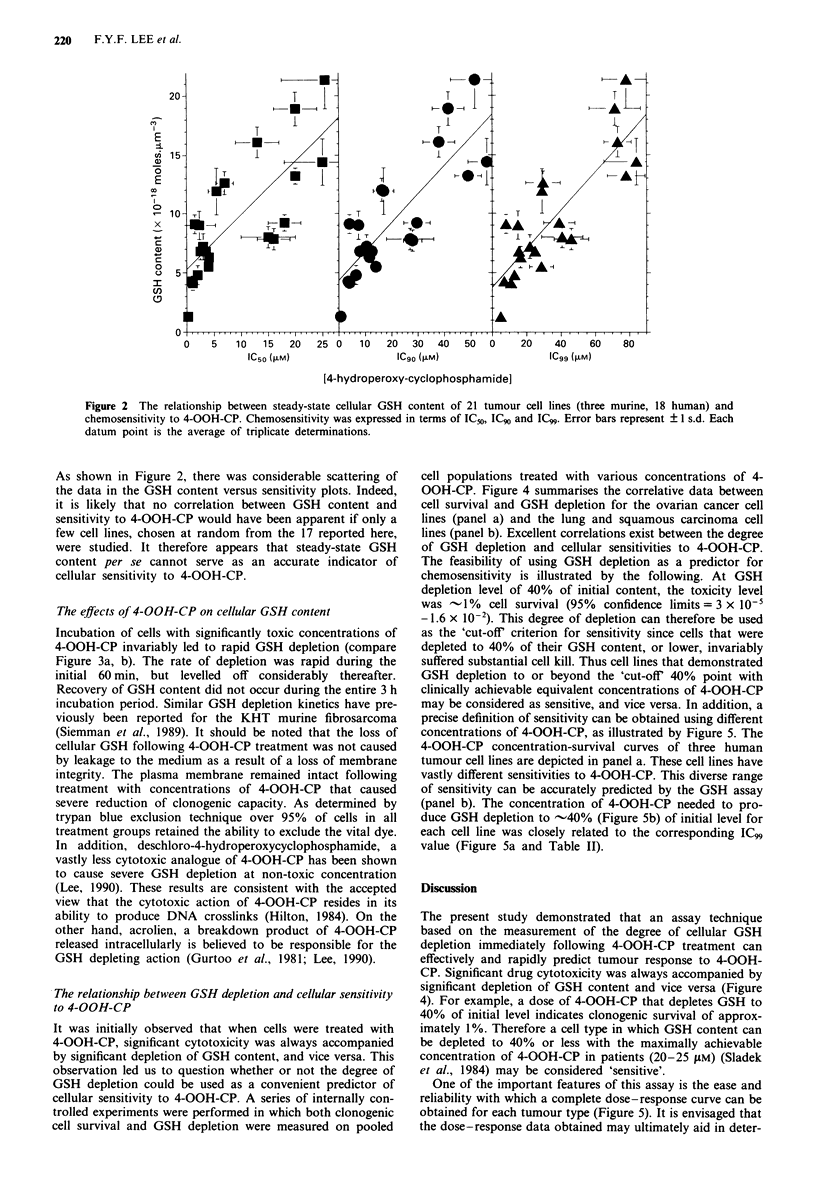
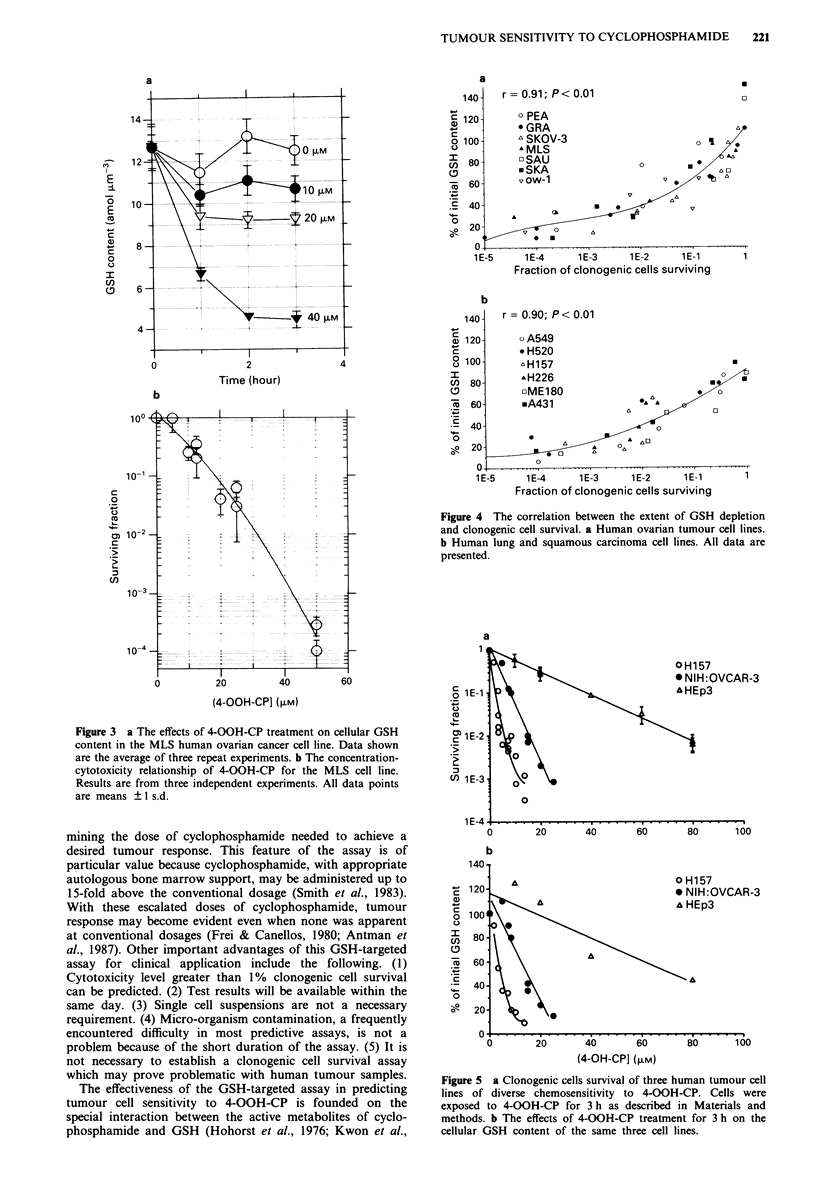
In an attempt to develop an assay to predict patient tumour response to cyclophosphamide (CP), the feasibility of using a glutathione-targeted assay to assess the in vitro chemosensitivity of tumour cells to 4-hydroperoxycyclophosphamide (4-OOH-CP), an activated congener of CP, was evaluated. A panel of 19 human and three murine tumour cell lines was used. These consisted of three main categories of tumour types, viz. ovarian, lung and squamous cell carcinoma. The major finding was that the occurrence of a significant reduction of tumour cell reproductive capacity was always accompanied by substantial depletion of cellular glutathione (GSH) content, and vice versa. Plots of % GSH depletion versus clonogenic cell survival demonstrated highly significant correlation (r = 0.90-0.91; P less than 0.01). It was determined that for in vitro tumour cell lines, a GSH depletion to 40% of initial content may serve as a cut-off criterion for chemosensitivity to 4-OOH-CP. This degree of GSH depletion is indicative of clonogenic cell survival of approximately 1% (95% confidence limits = 3 x 10(-5)-1.6 x 10(-2)). The relationship between steady state GSH content and intrinsic sensitivity to 4-OOH-CP was also evaluated. The GSH concentration of the tumour cell lines ranged from 1.3-21.2 x 10(-18) moles microns-3; chemosensitivity to 4-OOH-CP, in terms of IC99, was in the range of 5.0-87.1 microM. A good correlation was observed between these two parameters (r = 0.85, P less than 0.02). These results suggest that GSH plays an important role in determining the therapeutic efficacy of 4-OOH-CP in the treatment of cancer. It is uncertain, however, whether a high tumour steady state GSH content in itself is sufficient to cause therapeutic failure in patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams K. J., Carmichael J., Wolf C. R. Altered mouse bone marrow glutathione and glutathione transferase levels in response to cytotoxins. Cancer Res. 1985 Apr;45(4):1669–1673. [PubMed] [Google Scholar]
- Carmichael J., Friedman N., Tochner Z., Adams D. J., Wolf C. R., Mitchell J. B., Russo A. Inhibition of the protective effect of cyclophosphamide by pre-treatment with buthionine sulfoximine. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1191–1193. doi: 10.1016/0360-3016(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Crook T. R., Souhami R. L., Whyman G. D., McLean A. E. Glutathione depletion as a determinant of sensitivity of human leukemia cells to cyclophosphamide. Cancer Res. 1986 Oct;46(10):5035–5038. [PubMed] [Google Scholar]
- Frei E., 3rd, Canellos G. P. Dose: a critical factor in cancer chemotherapy. Am J Med. 1980 Oct;69(4):585–594. doi: 10.1016/0002-9343(80)90472-6. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Gurtoo H. L., Hipkens J. H., Sharma S. D. Role of glutathione in the metabolism-dependent toxicity and chemotherapy of cyclophosphamide. Cancer Res. 1981 Sep;41(9 Pt 1):3584–3591. [PubMed] [Google Scholar]
- Hilton J. Deoxyribonucleic acid crosslinking by 4-hydroperoxycyclophosphamide in cyclophosphamide-sensitive and -resistant L1210 cells. Biochem Pharmacol. 1984 Jun 15;33(12):1867–1872. doi: 10.1016/0006-2952(84)90541-0. [DOI] [PubMed] [Google Scholar]
- Hohorst H. J., Draeger U., Peter G., Voelcker G. The problem of oncostatic specificity of cyclophosphamide (NSC-26271): Studies on reactions that control the alkylating and cytotoxic activity. Cancer Treat Rep. 1976 Apr;60(4):309–315. [PubMed] [Google Scholar]
- Kallman R. F., Silini G., Van Putten L. M. Factors influencing the quantitative estimation of the in vivo survival of cells from solid tumors. J Natl Cancer Inst. 1967 Sep;39(3):539–549. [PubMed] [Google Scholar]
- Kwon C. H., Borch R. F., Engel J., Niemeyer U. Activation mechanisms of mafosfamide and the role of thiols in cyclophosphamide metabolism. J Med Chem. 1987 Feb;30(2):395–399. doi: 10.1021/jm00385a023. [DOI] [PubMed] [Google Scholar]
- Lee F. Y., Siemann D. W., Allalunis-Turner M. J., Keng P. C. Glutathione contents in human and rodent tumor cells in various phases of the cell cycle. Cancer Res. 1988 Jul 1;48(13):3661–3665. [PubMed] [Google Scholar]
- Lee F. Y., Siemann D. W., Sutherland R. M. Changes in cellular glutathione content during adriamycin treatment in human ovarian cancer--a possible indicator of chemosensitivity. Br J Cancer. 1989 Sep;60(3):291–298. doi: 10.1038/bjc.1989.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann D. W., Flaherty A. A., Penney D. P. Effect of thiol manipulation on chemopotentiation by nitroimidazoles. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1341–1345. doi: 10.1016/0360-3016(89)90311-8. [DOI] [PubMed] [Google Scholar]
- Sladek N. E., Doeden D., Powers J. F., Krivit W. Plasma concentrations of 4-hydroxycyclophosphamide and phosphoramide mustard in patients repeatedly given high doses of cyclophosphamide in preparation for bone marrow transplantation. Cancer Treat Rep. 1984 Oct;68(10):1247–1254. [PubMed] [Google Scholar]
- Smith I. E., Evans B. D., Harland S. J., Millar J. L. Autologous bone marrow rescue is unnecessary after very-high-dose cyclophosphamide. Lancet. 1983 Jan 1;1(8314-5):76–77. doi: 10.1016/s0140-6736(83)91617-3. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Whitescarver J., Jernstrom P., Nolan J. F., Byatt P. Some properties of a new epithelial cell line of human origin. J Natl Cancer Inst. 1970 Jul;45(1):107–122. [PubMed] [Google Scholar]
- TOOLAN H. W. Transplantable human neoplasms maintained in cortisone-treated laboratory animals: H.S. No. 1; H.Ep. No. 1; H.Ep. No. 2; H.Ep. No. 3; and H.Emb.Rh. No. 1. Cancer Res. 1954 Oct;14(9):660–666. [PubMed] [Google Scholar]
- Whillans D. W., Rauth A. M. An experimental and analytical study of oxygen depletion in stirred cell suspensions. Radiat Res. 1980 Oct;84(1):97–114. [PubMed] [Google Scholar]


