Abstract
Pseudomonas sp. strain JS42 utilizes 2-nitrotoluene (2NT) as the sole source of carbon and energy for growth. Intact cells catalyze the oxidation of 2NT to 3-methylcatechol and nitrite in a reaction that requires molecular oxygen. Cell extracts oxidized 2NT to 3-methylcatechol and nitrite in the presence of NAD(P)H and ferrous iron. Ion-exchange chromatography yielded three protein fractions (A, B, and C) which were all required for the oxidation of 2NT to 3-methylcatechol and nitrite. Component B (reductase2NT) catalyzed a NAD(P)H-dependent reduction of cytochrome c. Solutions of component A (ISP2NT) were brown and showed absorption maxima at 458 and 324 nm. Two major bands with M(r)s 52,500 and 28,000 were observed when ISP2NT was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Component C could be replaced by ferredoxin NAP from the Pseudomonas putida NCIB 9816-4 naphthalene dioxygenase system and was given the designation ferredoxin2NT. Experiments with 18O2 showed that both oxygen atoms were added to the aromatic ring of 2NT to yield 3-methylcatechol. The enzyme is a new multicomponent enzyme system which we have designated 2NT 2,3-dioxygenase.
Full text
PDF

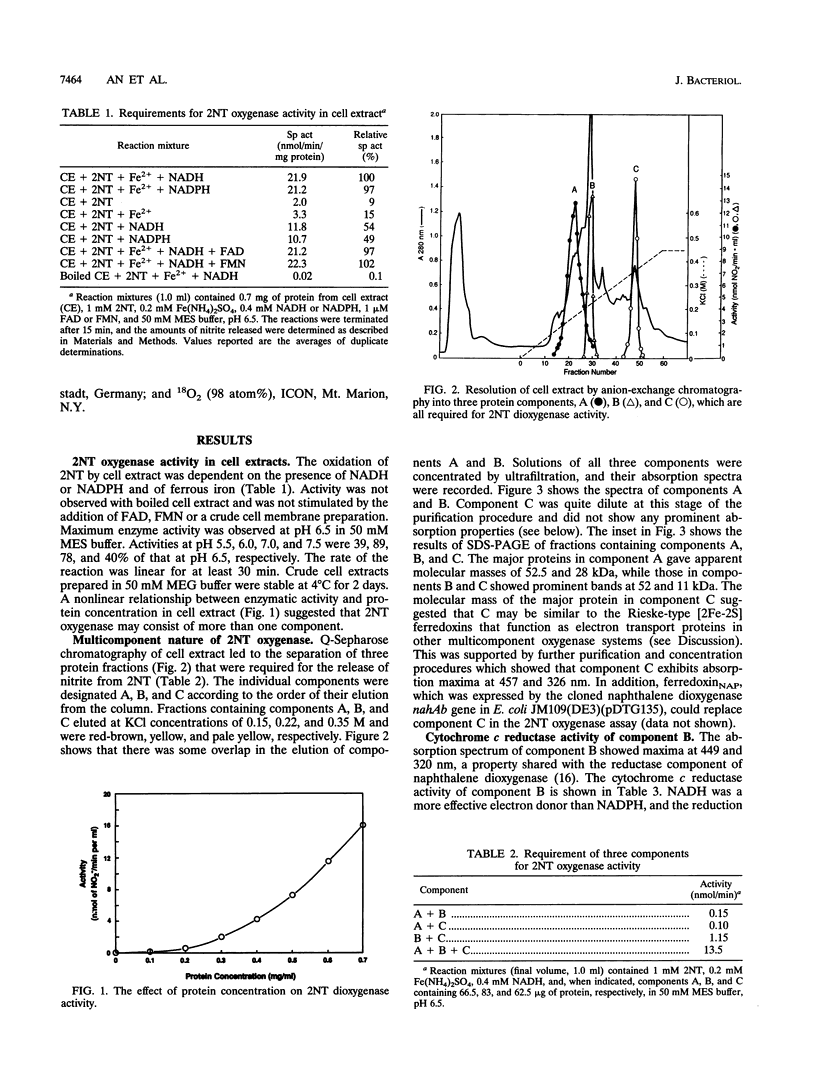
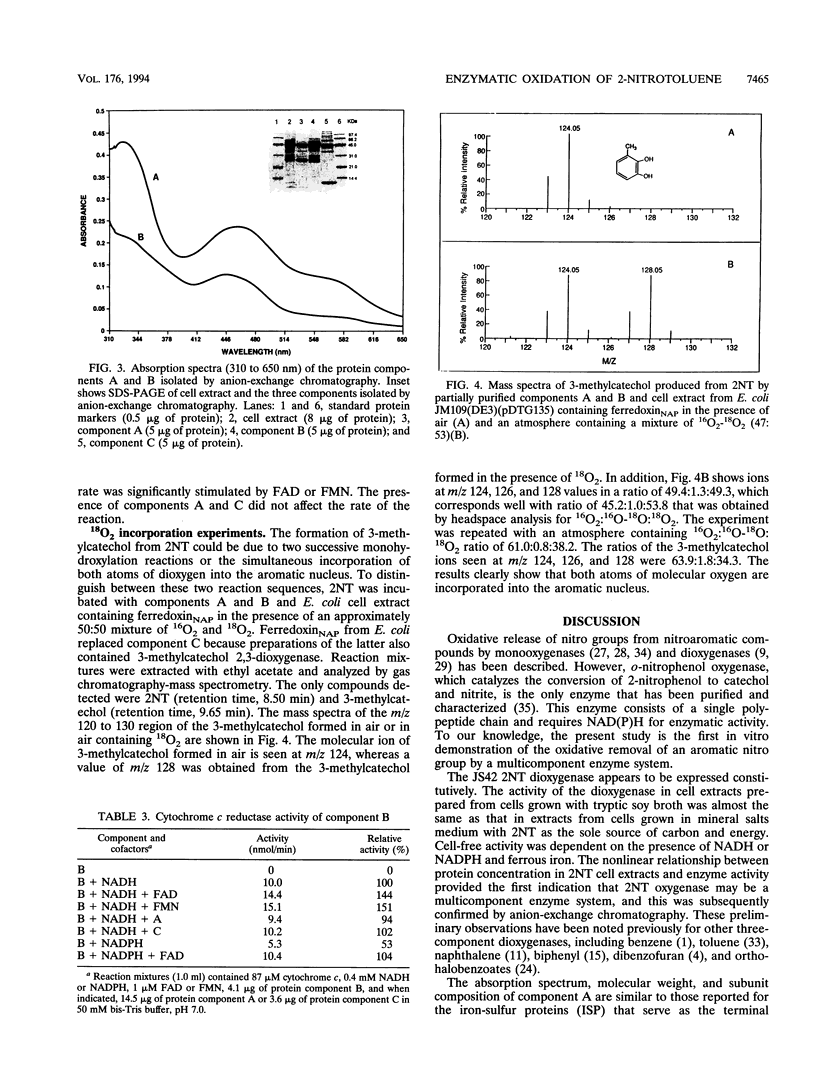
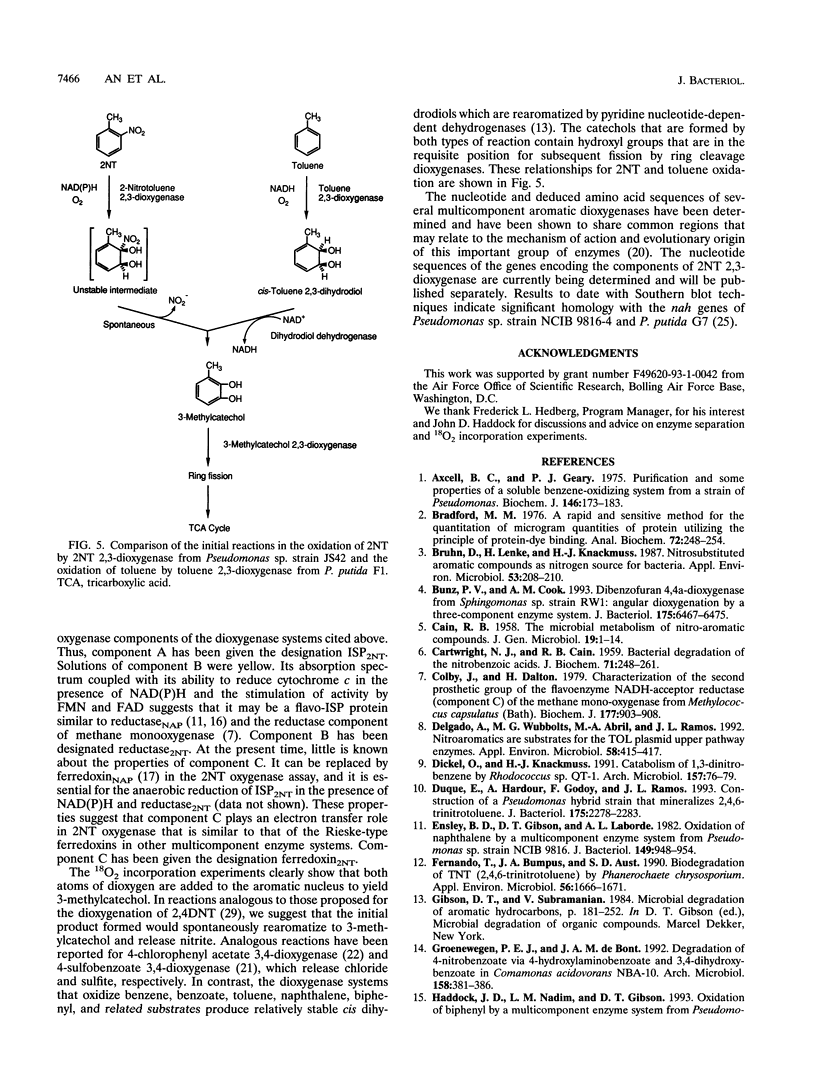

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axcell B. C., Geary P. J. Purification and some properties of a soluble benzene-oxidizing system from a strain of Pseudomonas. Biochem J. 1975 Jan;146(1):173–183. doi: 10.1042/bj1460173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruhn C., Lenke H., Knackmuss H. J. Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl Environ Microbiol. 1987 Jan;53(1):208–210. doi: 10.1128/aem.53.1.208-210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünz P. V., Cook A. M. Dibenzofuran 4,4a-dioxygenase from Sphingomonas sp. strain RW1: angular dioxygenation by a three-component enzyme system. J Bacteriol. 1993 Oct;175(20):6467–6475. doi: 10.1128/jb.175.20.6467-6475.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIN R. B. The microbial metabolism of nitro-aromatic compounds. J Gen Microbiol. 1958 Aug;19(1):1–14. doi: 10.1099/00221287-19-1-1. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT N. J., CAIN R. B. Bacterial degradation of the nitrobenzoic acids. Biochem J. 1959 Feb;71(2):248–261. doi: 10.1042/bj0710248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Characterization of the second prosthetic group of the flavoenzyme NADH-acceptor reductase (component C) of the methane mono-oxygenase from Methylococcus capsulatus (Bath). Biochem J. 1979 Mar 1;177(3):903–908. doi: 10.1042/bj1770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado A., Wubbolts M. G., Abril M. A., Ramos J. L. Nitroaromatics Are Substrates for the TOL Plasmid Upper-Pathway Enzymes. Appl Environ Microbiol. 1992 Jan;58(1):415–417. doi: 10.1128/aem.58.1.415-417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickel O., Knackmuss H. J. Catabolism of 1,3-dinitrobenzene by Rhodococcus sp. QT-1. Arch Microbiol. 1991;157(1):76–79. doi: 10.1007/BF00245339. [DOI] [PubMed] [Google Scholar]
- Duque E., Haidour A., Godoy F., Ramos J. L. Construction of a Pseudomonas hybrid strain that mineralizes 2,4,6-trinitrotoluene. J Bacteriol. 1993 Apr;175(8):2278–2283. doi: 10.1128/jb.175.8.2278-2283.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T., Laborde A. L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982 Mar;149(3):948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando T., Bumpus J. A., Aust S. D. Biodegradation of TNT (2,4,6-trinitrotoluene) by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Jun;56(6):1666–1671. doi: 10.1128/aem.56.6.1666-1671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock J. D., Nadim L. M., Gibson D. T. Oxidation of biphenyl by a multicomponent enzyme system from Pseudomonas sp. strain LB400. J Bacteriol. 1993 Jan;175(2):395–400. doi: 10.1128/jb.175.2.395-400.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of ferredoxinNAP, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):465–468. doi: 10.1128/jb.172.1.465-468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Spain J. C. Biodegradation of 4-nitrotoluene by Pseudomonas sp. strain 4NT. Appl Environ Microbiol. 1993 Jul;59(7):2239–2243. doi: 10.1128/aem.59.7.2239-2243.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Wallace W. H., Spain J. C. Biodegradation of 2-nitrotoluene by Pseudomonas sp. strain JS42. Appl Environ Microbiol. 1994 Sep;60(9):3466–3469. doi: 10.1128/aem.60.9.3466-3469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Kok M., Neidle E. L. Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol. 1992;46:565–601. doi: 10.1146/annurev.mi.46.100192.003025. [DOI] [PubMed] [Google Scholar]
- Locher H. H., Leisinger T., Cook A. M. 4-Sulphobenzoate 3,4-dioxygenase. Purification and properties of a desulphonative two-component enzyme system from Comamonas testosteroni T-2. Biochem J. 1991 Mar 15;274(Pt 3):833–842. doi: 10.1042/bj2740833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A., Klages U., Krauss S., Lingens F. Oxidation and dehalogenation of 4-chlorophenylacetate by a two-component enzyme system from Pseudomonas sp. strain CBS3. J Bacteriol. 1984 Nov;160(2):618–621. doi: 10.1128/jb.160.2.618-621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. B., Spain J. C., Haddock J. D., Gibson D. T. Oxidation of nitrotoluenes by toluene dioxygenase: evidence for a monooxygenase reaction. Appl Environ Microbiol. 1992 Aug;58(8):2643–2648. doi: 10.1128/aem.58.8.2643-2648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov V., Hausinger R. P. Pseudomonas aeruginosa 142 uses a three-component ortho-halobenzoate 1,2-dioxygenase for metabolism of 2,4-dichloro- and 2-chlorobenzoate. J Bacteriol. 1994 Jun;176(11):3368–3374. doi: 10.1128/jb.176.11.3368-3374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. J., Osslund T. D., Saunders R., Ensley B. D., Suggs S., Harcourt A., Suen W. C., Cruden D. L., Gibson D. T., Zylstra G. J. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816-4. Gene. 1993 May 15;127(1):31–37. doi: 10.1016/0378-1119(93)90613-8. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Gibson D. T. Pathway for Biodegradation of p-Nitrophenol in a Moraxella sp. Appl Environ Microbiol. 1991 Mar;57(3):812–819. doi: 10.1128/aem.57.3.812-819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Wyss O., Gibson D. T. Enzymatic oxidation of p-nitrophenol. Biochem Biophys Res Commun. 1979 May 28;88(2):634–641. doi: 10.1016/0006-291x(79)92095-3. [DOI] [PubMed] [Google Scholar]
- Spanggord R. J., Spain J. C., Nishino S. F., Mortelmans K. E. Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Appl Environ Microbiol. 1991 Nov;57(11):3200–3205. doi: 10.1128/aem.57.11.3200-3205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen W. C., Spain J. C. Cloning and characterization of Pseudomonas sp. strain DNT genes for 2,4-dinitrotoluene degradation. J Bacteriol. 1993 Mar;175(6):1831–1837. doi: 10.1128/jb.175.6.1831-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh W. K., Gibson D. T., Liu T. N. Toluene dioxygenase: a multicomponent enzyme system. Biochem Biophys Res Commun. 1977 Sep 9;78(1):401–410. doi: 10.1016/0006-291x(77)91268-2. [DOI] [PubMed] [Google Scholar]
- Zeyer J., Kocher H. P. Purification and characterization of a bacterial nitrophenol oxygenase which converts ortho-nitrophenol to catechol and nitrite. J Bacteriol. 1988 Apr;170(4):1789–1794. doi: 10.1128/jb.170.4.1789-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]