Abstract
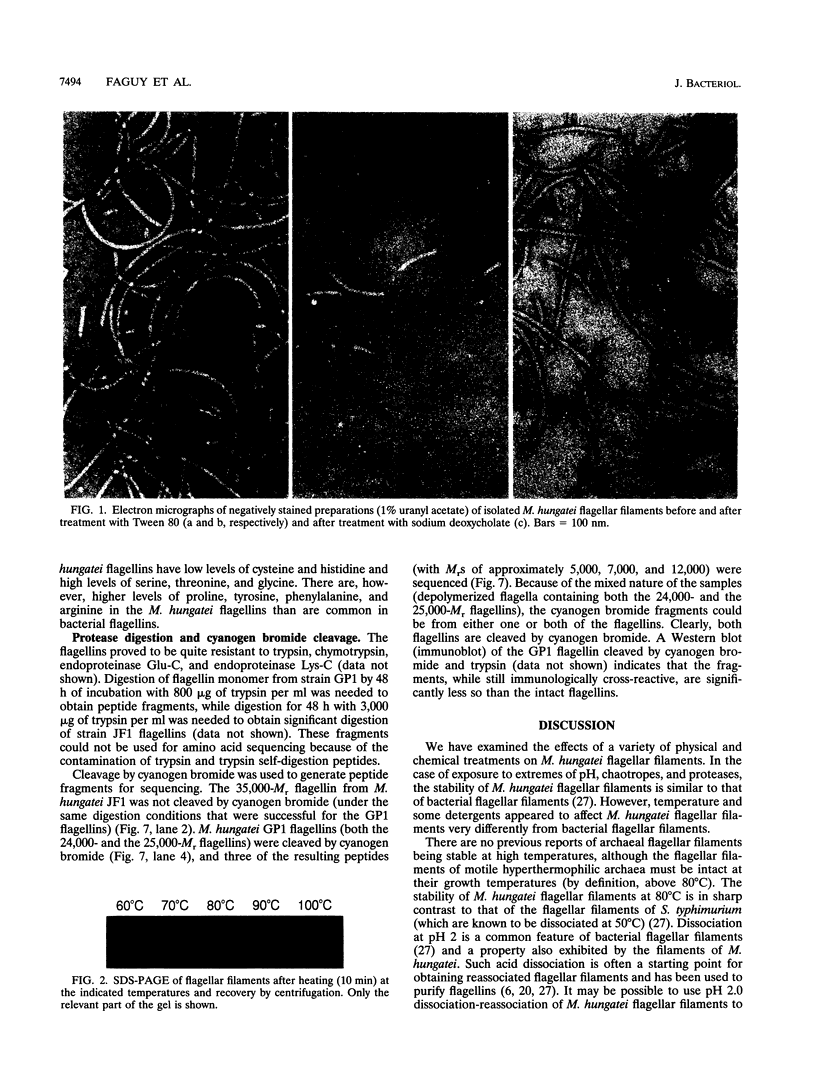
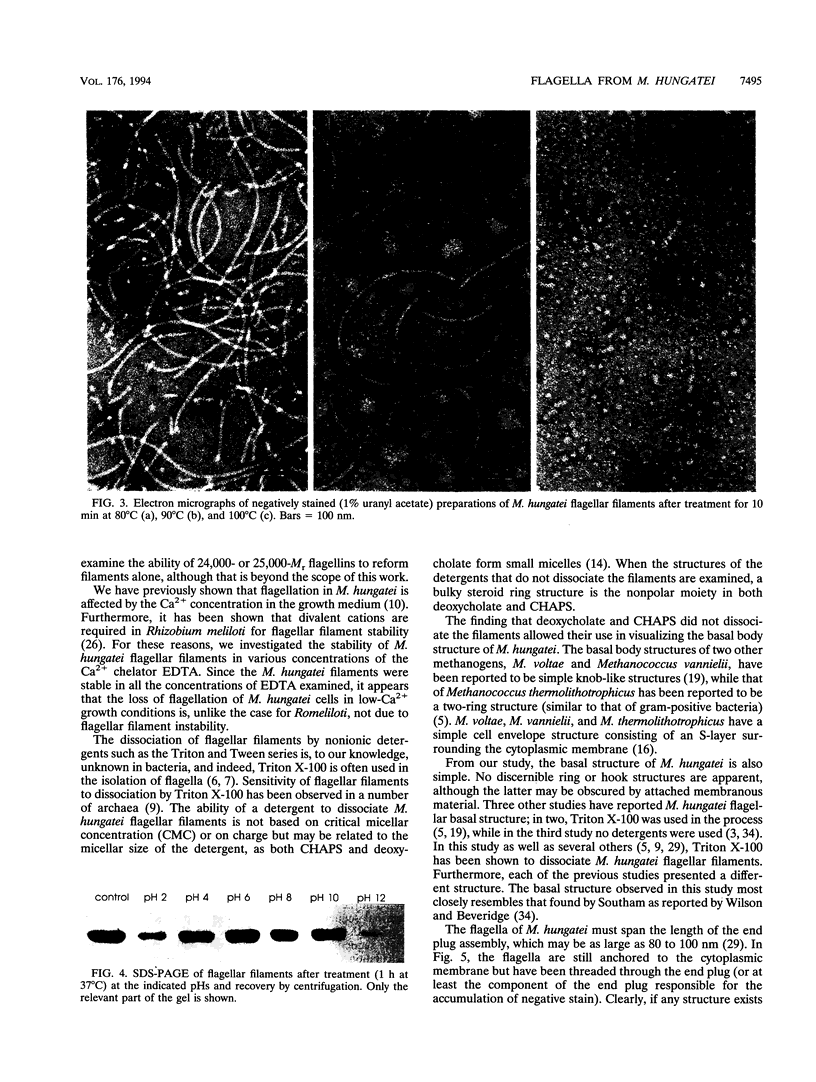
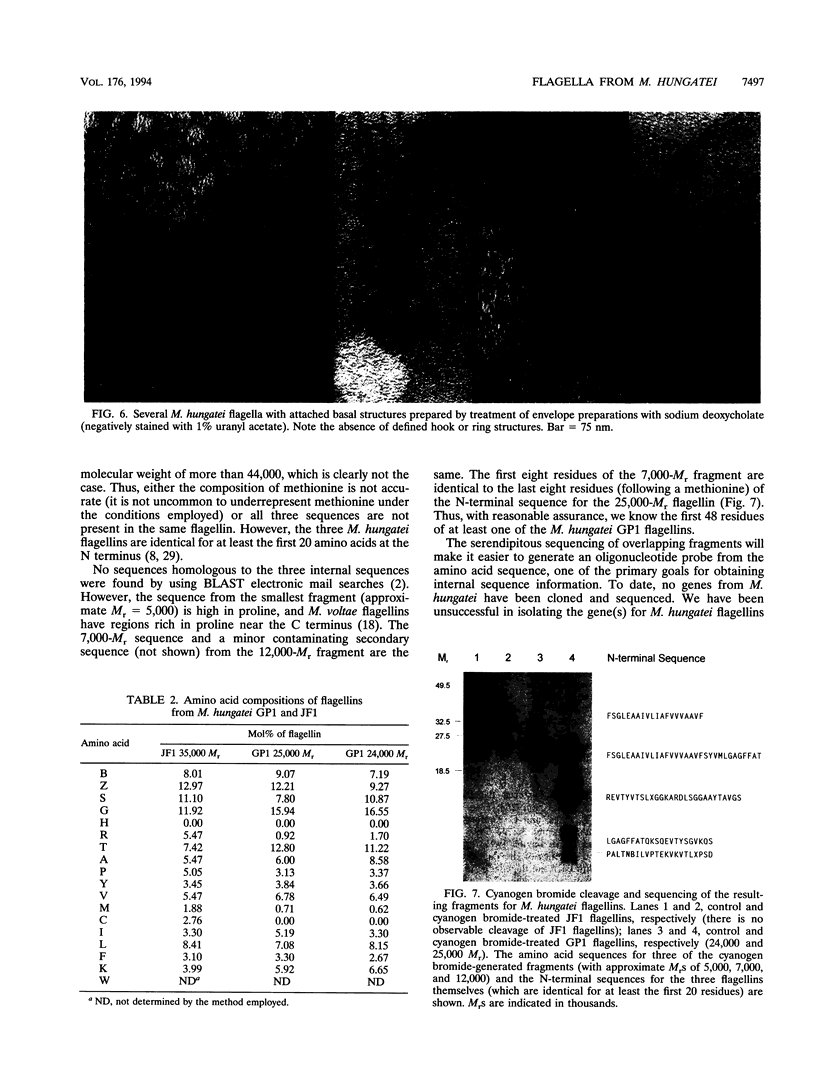
Flagellar filaments from Methanospirillum hungatei GP1 and JF1 were isolated and subjected to a variety of physical and chemical treatments. The filaments were stable to temperatures up to 80 degrees C and over the pH range of 4 to 10. The flagellar filaments were dissociated in the detergents (final concentration of 0.5%) Triton X-100, Tween 20, Tween 80, Brij 58, N-octylglucoside, cetyltrimethylammonium bromide, and Zwittergent 3-14, remaining intact in only two of the detergents tested, sodium deoxycholate and 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate (CHAPS). Spheroplasting techniques were used to separate the internal cells from the complex sheath, S-layer (cell wall), and end plugs of M. hungatei. The flagellar basal structure was visualized after solubilization of membranes by CHAPS or deoxycholate. The basal structure appeared to be a simple knob with no apparent ring or hook structures. The multiple, glycosylated flagellins constituting the flagellar filaments were cleaved by proteases and cyanogen bromide. The cyanogen bromide-generated fragments of M. hungatei GP1 flagellins were partially sequenced to provide internal sequence information. In addition, the amino acid composition of each flagellin was determined and indicated that the flagellins are distinct gene products, rather than differentially glycosylated forms of the same gene product.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa S. I., Vonderviszt F., Ishima R., Akasaka K. Termini of Salmonella flagellin are disordered and become organized upon polymerization into flagellar filament. J Mol Biol. 1990 Feb 20;211(4):673–677. doi: 10.1016/0022-2836(90)90064-S. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Stewart M., Doyle R. J., Sprott G. D. Unusual stability of the Methanospirillum hungatei sheath. J Bacteriol. 1985 May;162(2):728–737. doi: 10.1128/jb.162.2.728-737.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden D., Sparling R., Markovetz A. J. Isolation and Ultrastructure of the Flagella of Methanococcus thermolithotrophicus and Methanospirillum hungatei. Appl Environ Microbiol. 1989 Jun;55(6):1414–1419. doi: 10.1128/aem.55.6.1414-1419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Fine structure and isolation of the hook-basal body complex of flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):384–395. doi: 10.1128/jb.105.1.384-395.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driks A., DeRosier D. J. Additional structures associated with bacterial flagellar basal body. J Mol Biol. 1990 Feb 20;211(4):669–672. doi: 10.1016/0022-2836(90)90063-R. [DOI] [PubMed] [Google Scholar]
- Faguy D. M., Jarrell K. F., Kuzio J., Kalmokoff M. L. Molecular analysis of archael flagellins: similarity to the type IV pilin-transport superfamily widespread in bacteria. Can J Microbiol. 1994 Jan;40(1):67–71. doi: 10.1139/m94-011. [DOI] [PubMed] [Google Scholar]
- Faguy D. M., Koval S. F., Jarrell K. F. Correlation between glycosylation of flagellin proteins and sensitivity of flagellar filaments to Triton X-100 in methanogens. FEMS Microbiol Lett. 1992 Jan 1;69(2):129–134. doi: 10.1016/0378-1097(92)90616-v. [DOI] [PubMed] [Google Scholar]
- Firtel M., Southam G., Harauz G., Beveridge T. J. Characterization of the cell wall of the sheathed methanogen Methanospirillum hungatei GP1 as an S layer. J Bacteriol. 1993 Dec;175(23):7550–7560. doi: 10.1128/jb.175.23.7550-7560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerl L., Sumper M. Halobacterial flagellins are encoded by a multigene family. Characterization of five flagellin genes. J Biol Chem. 1988 Sep 15;263(26):13246–13251. [PubMed] [Google Scholar]
- Helenius A., McCaslin D. R., Fries E., Tanford C. Properties of detergents. Methods Enzymol. 1979;56:734–749. doi: 10.1016/0076-6879(79)56066-2. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Koval S. F. Ultrastructure and biochemistry of Methanococcus voltae. Crit Rev Microbiol. 1989;17(1):53–87. doi: 10.3109/10408418909105722. [DOI] [PubMed] [Google Scholar]
- Jones C. J., Aizawa S. The bacterial flagellum and flagellar motor: structure, assembly and function. Adv Microb Physiol. 1991;32:109–172. doi: 10.1016/s0065-2911(08)60007-7. [DOI] [PubMed] [Google Scholar]
- Kalmokoff M. L., Jarrell K. F. Cloning and sequencing of a multigene family encoding the flagellins of Methanococcus voltae. J Bacteriol. 1991 Nov;173(22):7113–7125. doi: 10.1128/jb.173.22.7113-7125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmokoff M. L., Jarrell K. F., Koval S. F. Isolation of flagella from the archaebacterium Methanococcus voltae by phase separation with Triton X-114. J Bacteriol. 1988 Apr;170(4):1752–1758. doi: 10.1128/jb.170.4.1752-1758.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. Gene to ultrastructure: the case of the flagellar basal body. J Bacteriol. 1993 Apr;175(8):2169–2174. doi: 10.1128/jb.175.8.2169-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyukova A. S., Pyatibratov M. G., Filimonov V. V., Fedorov O. V. Flagellin parts acquiring a regular structure during polymerization are disposed on the molecule ends. FEBS Lett. 1988 Dec 5;241(1-2):141–144. doi: 10.1016/0014-5793(88)81047-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- Patel G. B., Roth L. A., van den Berg L., Clark D. S. Characterization of a strain of Methanospirillum hungatti. Can J Microbiol. 1976 Sep;22(9):1404–1410. doi: 10.1139/m76-208. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Tuovinen O. H., Bauer W. D. Role of divalent cations in the subunit associations of complex flagella from Rhizobium meliloti. J Bacteriol. 1992 Jun;174(12):3896–3902. doi: 10.1128/jb.174.12.3896-3902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. I. Bacterial flagella. Annu Rev Microbiol. 1977;31:397–419. doi: 10.1146/annurev.mi.31.100177.002145. [DOI] [PubMed] [Google Scholar]
- Southam G., Firtel M., Blackford B. L., Jericho M. H., Xu W., Mulhern P. J., Beveridge T. J. Transmission electron microscopy, scanning tunneling microscopy, and atomic force microscopy of the cell envelope layers of the archaeobacterium Methanospirillum hungatei GP1. J Bacteriol. 1993 Apr;175(7):1946–1955. doi: 10.1128/jb.175.7.1946-1955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam G., Kalmokoff M. L., Jarrell K. F., Koval S. F., Beveridge T. J. Isolation, characterization, and cellular insertion of the flagella from two strains of the archaebacterium Methanospirillum hungatei. J Bacteriol. 1990 Jun;172(6):3221–3228. doi: 10.1128/jb.172.6.3221-3228.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., Colvin J. R., McKellar R. C. Spheroplasts of Methanospirillum hungatii formed upon treatment with dithiothreitol. Can J Microbiol. 1979 Jun;25(6):730–738. doi: 10.1139/m79-106. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Shaw K. M., Jarrell K. F. Isolation and chemical composition of the cytoplasmic membrane of the archaebacterium Methanospirillum hungatei. J Biol Chem. 1983 Mar 25;258(6):4026–4031. [PubMed] [Google Scholar]
- Sumper M. Halobacterial glycoprotein biosynthesis. Biochim Biophys Acta. 1987 Apr 27;906(1):69–79. doi: 10.1016/0304-4157(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Beveridge T. J. Bacterial flagellar filaments and their component flagellins. Can J Microbiol. 1993 May;39(5):451–472. doi: 10.1139/m93-066. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]