Abstract
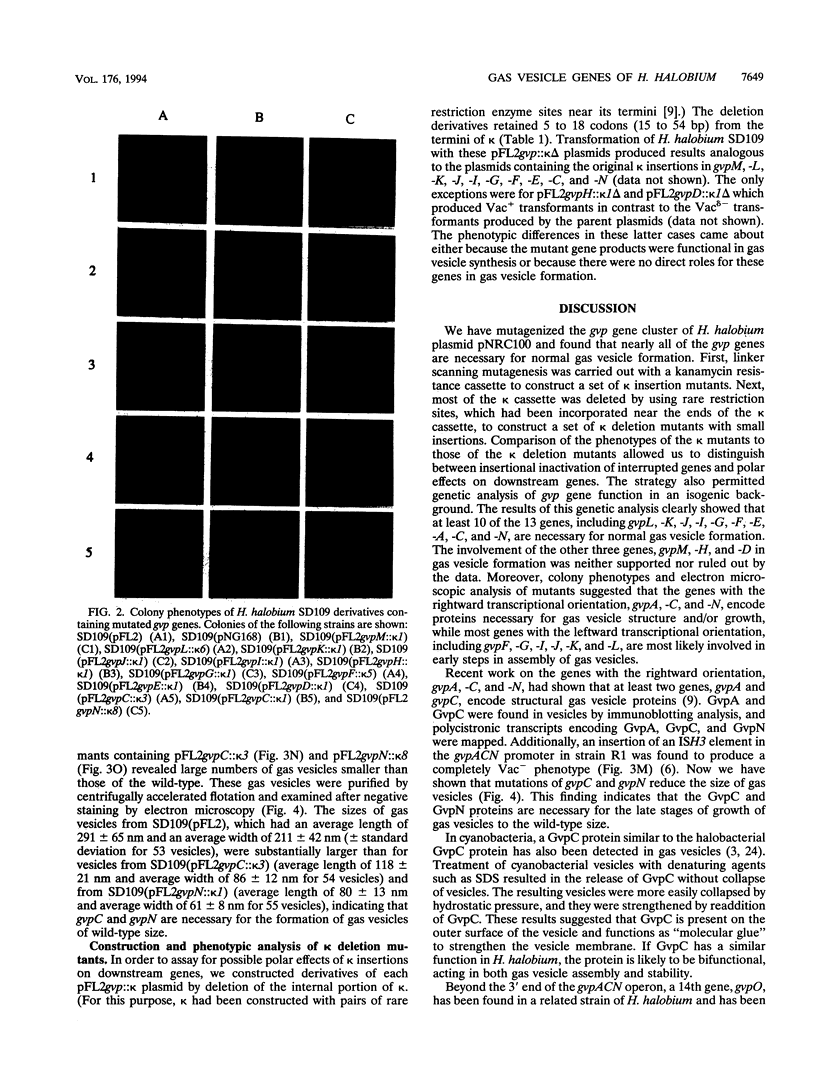
To study the functions of the 13 gvp genes, gvpMLKJIHGFEDACN, on plasmid pNRC100 of Halobacterium halobium in gas vesicle formation, we carried out linker scanning mutagenesis of the gene cluster. We constructed a 24.5-kb Escherichia coli-H. halobium shuttle plasmid, pFL2, containing the gvp gene cluster and introduced a kanamycin resistance (kappa) cassette into each gene (except for gvpA). Transformation of H. halobium SD109, which had the entire gvp gene cluster deleted, with pFL2 and mutated pFL2 derivatives showed that while the unmutated gene cluster successfully programmed gas vesicle formation, derivatives with insertion of the kappa cassette in any of the gvp genes, except gvpM, did not lead to production of normal gas vesicles. Insertions in gvpL, -K, -J, -I, and -F resulted in a complete block in gas vesicle synthesis, while insertions in gvpH, -G, -E, -D, -C, and -N resulted in greatly reduced gas vesicle synthesis. In most cases, the block in gas vesicle synthesis did not result from polar effects, since similar results were obtained for derivatives of the insertion mutants in which most of the internal portion of the kappa cassette was deleted and only small (15 to 54-bp) insertions remained. The only exceptions were for gvpH and gvpD, where deletion of the internal portion of the kappa insertions resulted in phenotypic reversion. Electron microscopic analysis of the kappa mutants revealed that interruptions of gvpC and gvpN result in the formation of smaller gas vesicle than in the wild type, while interruptions of gvpF, -G, -H, -J, -K, and -L produce no discernible vesicle intermediates. These results indicate the gvpA, -C, and -N, which have the rightward transcriptional orientation, encode structural proteins, with gvpC and gvpN necessary for late stages of vesicle formation, and gvpL, -K, -J, -I, -H, -G, and -F, which have the leftward transcriptional orientation encode proteins involved in early steps in the assembly of gas vesicles.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Cline S. W., Doolittle W. F. Efficient transfection of the archaebacterium Halobacterium halobium. J Bacteriol. 1987 Mar;169(3):1341–1344. doi: 10.1128/jb.169.3.1341-1344.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerval T., Houmard J., Guglielmi G., Csiszar K., Tandeau de Marsac N. A developmentally regulated gvpABC operon is involved in the formation of gas vesicles in the cyanobacterium Calothrix 7601. Gene. 1987;54(1):83–92. doi: 10.1016/0378-1119(87)90350-7. [DOI] [PubMed] [Google Scholar]
- DasSarma S., Damerval T., Jones J. G., Tandeau de Marsac N. A plasmid-encoded gas vesicle protein gene in a halophilic archaebacterium. Mol Microbiol. 1987 Nov;1(3):365–370. doi: 10.1111/j.1365-2958.1987.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Dassarma S., Halladay J. T., Jones J. G., Donovan J. W., Giannasca P. J., de Marsac N. T. High-frequency mutations in a plasmid-encoded gas vesicle gene in Halobacterium halobium. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6861–6865. doi: 10.1073/pnas.85.18.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert C., Krüger K., Offner S., Pfeifer F. Three different but related gene clusters encoding gas vesicles in halophilic archaea. J Mol Biol. 1992 Sep 20;227(2):586–592. doi: 10.1016/0022-2836(92)90914-6. [DOI] [PubMed] [Google Scholar]
- Englert C., Pfeifer F. Analysis of gas vesicle gene expression in Haloferax mediterranei reveals that GvpA and GvpC are both gas vesicle structural proteins. J Biol Chem. 1993 May 5;268(13):9329–9336. [PubMed] [Google Scholar]
- Halladay J. T., Jones J. G., Lin F., MacDonald A. B., DasSarma S. The rightward gas vesicle operon in Halobacterium plasmid pNRC100: identification of the gvpA and gvpC gene products by use of antibody probes and genetic analysis of the region downstream of gvpC. J Bacteriol. 1993 Feb;175(3):684–692. doi: 10.1128/jb.175.3.684-692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay J. T., Ng W. L., DasSarma S. Genetic transformation of a halophilic archaebacterium with a gas vesicle gene cluster restores its ability to float. Gene. 1992 Sep 21;119(1):131–136. doi: 10.1016/0378-1119(92)90078-4. [DOI] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. G., Hackett N. R., Halladay J. T., Scothorn D. J., Yang C. F., Ng W. L., DasSarma S. Analysis of insertion mutants reveals two new genes in the pNRC100 gas vesicle gene cluster of Halobacterium halobium. Nucleic Acids Res. 1989 Oct 11;17(19):7785–7793. doi: 10.1093/nar/17.19.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. G., Young D. C., DasSarma S. Structure and organization of the gas vesicle gene cluster on the Halobacterium halobium plasmid pNRC100. Gene. 1991 Jun 15;102(1):117–122. doi: 10.1016/0378-1119(91)90549-q. [DOI] [PubMed] [Google Scholar]
- Krantz M. J., Ballou C. E. Analysis of Halobacterium halobium gas vesicles. J Bacteriol. 1973 Jun;114(3):1058–1067. doi: 10.1128/jb.114.3.1058-1067.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam W. L., Doolittle W. F. Shuttle vectors for the archaebacterium Halobacterium volcanii. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5478–5482. doi: 10.1073/pnas.86.14.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. L., DasSarma S. Minimal replication origin of the 200-kilobase Halobacterium plasmid pNRC100. J Bacteriol. 1993 Aug;175(15):4584–4596. doi: 10.1128/jb.175.15.4584-4596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. L., Kothakota S., DasSarma S. Structure of the gas vesicle plasmid in Halobacterium halobium: inversion isomers, inverted repeats, and insertion sequences. J Bacteriol. 1991 Mar;173(6):1958–1964. doi: 10.1128/jb.173.6.1958-1964.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Walsby A. E. Gas vesicles. Microbiol Rev. 1994 Mar;58(1):94–144. doi: 10.1128/mr.58.1.94-144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. F., DasSarma S. Transcriptional induction of purple membrane and gas vesicle synthesis in the archaebacterium Halobacterium halobium is blocked by a DNA gyrase inhibitor. J Bacteriol. 1990 Jul;172(7):4118–4121. doi: 10.1128/jb.172.7.4118-4121.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]