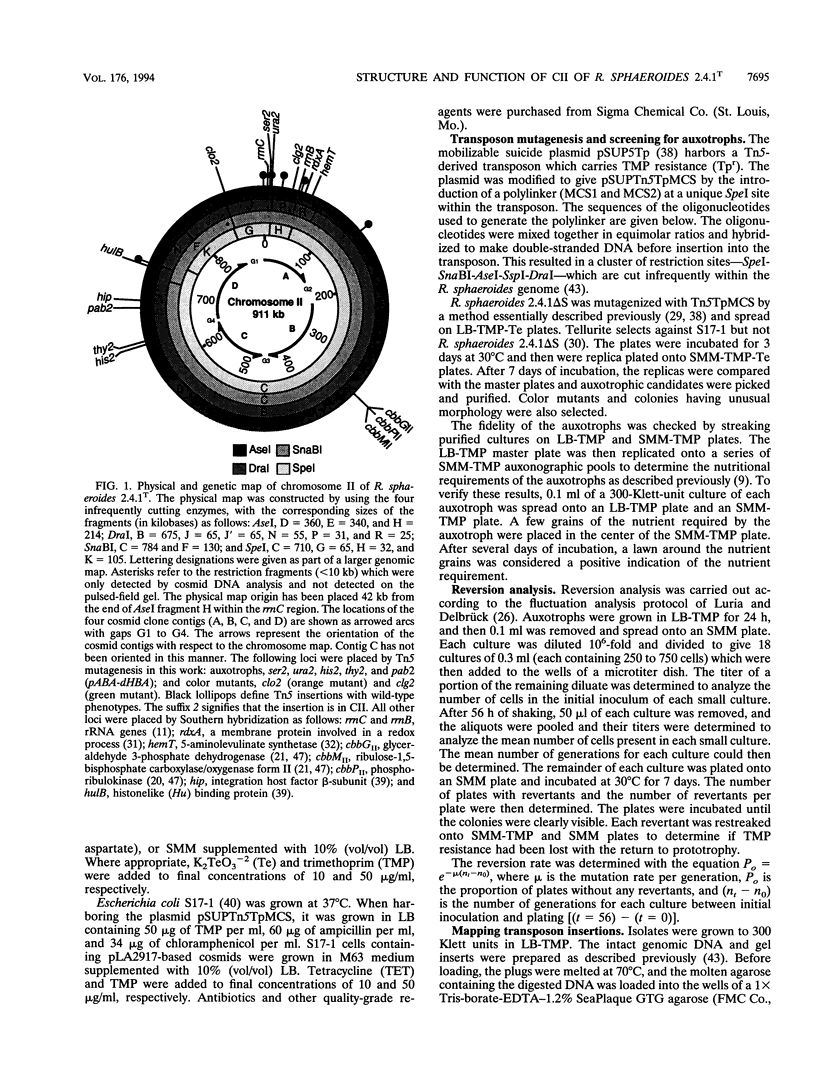
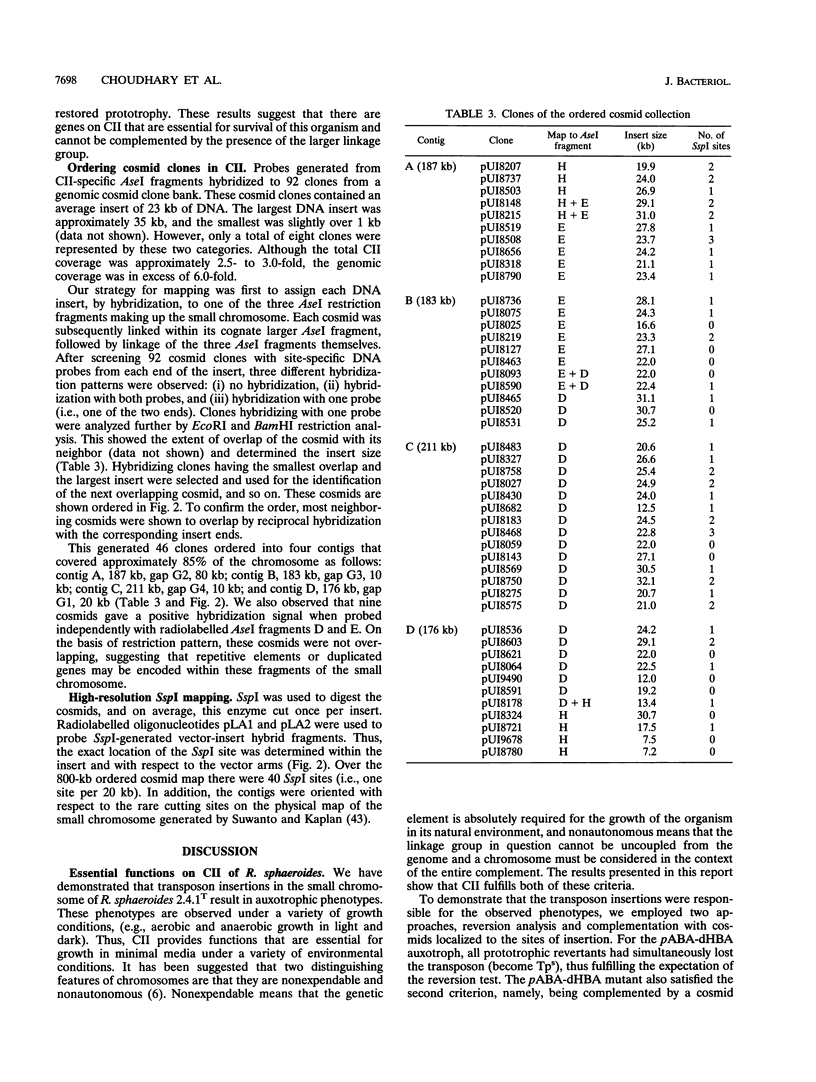
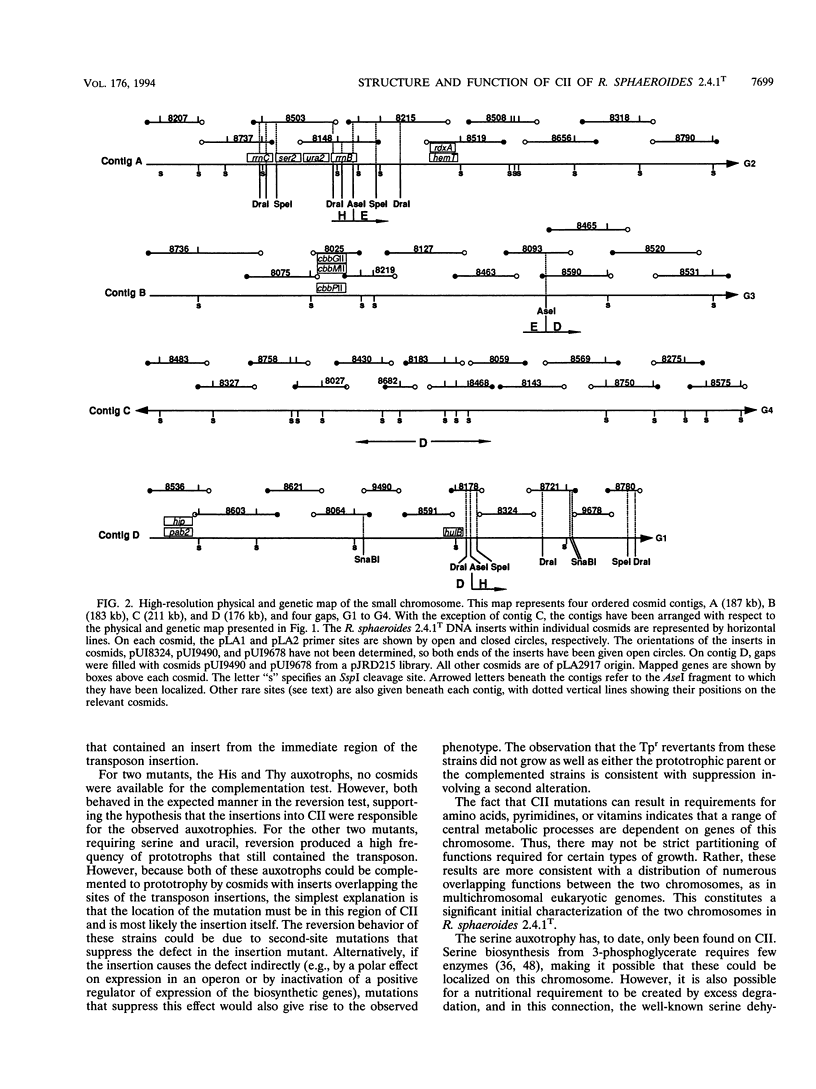
Abstract
Although multiple chromosomes occur in bacteria, much remains to be learned about their structural and functional interrelationships. To study the structure-function relationships of chromosomes I and II of the facultative photosynthetic bacterium Rhodobacter sphaeroides 2.4.1T, auxotrophic mutants were isolated. Five strains having transposon insertions in chromosome II showed requirements for p-aminobenzoic acid (pABA)-dihydroxybenzoic acid (dHBA), serine, thymine, uracil, or histidine. The His, Thy, and pABA-dHBA mutants reverted to prototrophy at low frequency and concordantly lost their transposon insertions from the genome. The Ser, Ura, and pABA-dHBA mutants were complemented by cosmids that carried the region of chromosome II where the transposon insertions were located. The cosmids used for complementation analysis were selected, on the basis of map position, from a set of overlapping clones that had been ordered by a combination of hybridization and restriction endonuclease mapping. These experiments provide the basis for detailed studies of the structure, function, and interaction between each chromosome, and they demonstrate at this early stage of investigation that no fundamental differences exist between each chromosome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Michaux-Charachon S., Jumas-Bilak E., Karayan L., Ramuz M. Presence of one linear and one circular chromosome in the Agrobacterium tumefaciens C58 genome. J Bacteriol. 1993 Dec;175(24):7869–7874. doi: 10.1128/jb.175.24.7869-7874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen L. N., Hanson R. S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985 Mar;161(3):955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo V., Alvarez E., Zumstein E., Damiani G., Sgaramella V., Ehrlich S. D., Serror P. An ordered collection of Bacillus subtilis DNA segments cloned in yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6047–6051. doi: 10.1073/pnas.90.13.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukanov N. O., Berg D. E. Ordered cosmid library and high-resolution physical-genetic map of Helicobacter pylori strain NCTC11638. Mol Microbiol. 1994 Feb;11(3):509–523. doi: 10.1111/j.1365-2958.1994.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Campbell A. Evolutionary significance of accessory DNA elements in bacteria. Annu Rev Microbiol. 1981;35:55–83. doi: 10.1146/annurev.mi.35.100181.000415. [DOI] [PubMed] [Google Scholar]
- Cheng H. P., Lessie T. G. Multiple replicons constituting the genome of Pseudomonas cepacia 17616. J Bacteriol. 1994 Jul;176(13):4034–4042. doi: 10.1128/jb.176.13.4034-4042.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi M., Messens E., Caplan A. B., van Montagu M., Desomer J. Fasciation induction by the phytopathogen Rhodococcus fascians depends upon a linear plasmid encoding a cytokinin synthase gene. EMBO J. 1992 Mar;11(3):795–804. doi: 10.1002/j.1460-2075.1992.tb05116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden S. C., Kaplan S. Identification of cis-acting regulatory regions upstream of the rRNA operons of Rhodobacter sphaeroides. J Bacteriol. 1993 Oct;175(20):6392–6402. doi: 10.1128/jb.175.20.6392-6402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden S. C., Kaplan S. Localization and structural analysis of the ribosomal RNA operons of Rhodobacter sphaeroides. Nucleic Acids Res. 1990 Dec 25;18(24):7267–7277. doi: 10.1093/nar/18.24.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiglmeier K., Honoré N., Woods S. A., Caudron B., Cole S. T. Use of an ordered cosmid library to deduce the genomic organization of Mycobacterium leprae. Mol Microbiol. 1993 Jan;7(2):197–206. doi: 10.1111/j.1365-2958.1993.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonstein M., Haselkorn R. Chromosomal structure of Rhodobacter capsulatus strain SB1003: cosmid encyclopedia and high-resolution physical and genetic map. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2522–2526. doi: 10.1073/pnas.90.6.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonstein M., Zheng S., Haselkorn R. Physical map of the genome of Rhodobacter capsulatus SB 1003. J Bacteriol. 1992 Jun;174(12):4070–4077. doi: 10.1128/jb.174.12.4070-4077.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari C. S., Watkins M., Kaplan S. Plasmid distribution and analyses in Rhodopseudomonas sphaeroides. Plasmid. 1984 Jan;11(1):39–47. doi: 10.1016/0147-619x(84)90005-2. [DOI] [PubMed] [Google Scholar]
- Grabowski R., Hofmeister A. E., Buckel W. Bacterial L-serine dehydratases: a new family of enzymes containing iron-sulfur clusters. Trends Biochem Sci. 1993 Aug;18(8):297–300. doi: 10.1016/0968-0004(93)90040-t. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. L., Lerchen R., Hessler P., Kaplan S. Phosphoribulokinase activity and regulation of CO2 fixation critical for photosynthetic growth of Rhodobacter sphaeroides. J Bacteriol. 1990 Apr;172(4):1749–1761. doi: 10.1128/jb.172.4.1749-1761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. L., Lerchen R., Hessler P., Kaplan S. Roles of CfxA, CfxB, and external electron acceptors in regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase expression in Rhodobacter sphaeroides. J Bacteriol. 1990 Apr;172(4):1736–1748. doi: 10.1128/jb.172.4.1736-1748.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusterspreute M., Ha Thi V., Emery S., Tournis-Gamble S., Kennedy N., Davison J. Vectors with restriction site banks. IV. pJRD184, a 3793-bp plasmid vector with 49 unique restriction sites. Gene. 1985;39(2-3):299–304. doi: 10.1016/0378-1119(85)90327-0. [DOI] [PubMed] [Google Scholar]
- Krawiec S., Riley M. Organization of the bacterial chromosome. Microbiol Rev. 1990 Dec;54(4):502–539. doi: 10.1128/mr.54.4.502-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Kieser H. M., Hopwood D. A., Chen C. W. The chromosomal DNA of Streptomyces lividans 66 is linear. Mol Microbiol. 1993 Dec;10(5):923–933. doi: 10.1111/j.1365-2958.1993.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaux S., Paillisson J., Carles-Nurit M. J., Bourg G., Allardet-Servent A., Ramuz M. Presence of two independent chromosomes in the Brucella melitensis 16M genome. J Bacteriol. 1993 Feb;175(3):701–705. doi: 10.1128/jb.175.3.701-705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. D., Kaplan S. Construction of TnphoA gene fusions in Rhodobacter sphaeroides: isolation and characterization of a respiratory mutant unable to utilize dimethyl sulfoxide as a terminal electron acceptor during anaerobic growth in the dark on glucose. J Bacteriol. 1989 Aug;171(8):4385–4394. doi: 10.1128/jb.171.8.4385-4394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. D., Kaplan S. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J Bacteriol. 1992 Mar;174(5):1505–1514. doi: 10.1128/jb.174.5.1505-1514.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Kaplan S. Expression of the Rhodobacter sphaeroides hemA and hemT genes, encoding two 5-aminolevulinic acid synthase isozymes. J Bacteriol. 1993 Apr;175(8):2292–2303. doi: 10.1128/jb.175.8.2292-2303.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Kaplan S. Rhodobacter sphaeroides rdxA, a homolog of Rhizobium meliloti fixG, encodes a membrane protein which may bind cytoplasmic [4Fe-4S] clusters. J Bacteriol. 1992 Oct;174(20):6444–6454. doi: 10.1128/jb.174.20.6444-6454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I. THE PATHWAY AND CONTROL OF SERINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Biol Chem. 1963 Dec;238:3934–3944. [PubMed] [Google Scholar]
- Pardo M. A., Lagunez J., Miranda J., Martínez E. Nodulating ability of Rhizobium tropici is conditioned by a plasmid-encoded citrate synthase. Mol Microbiol. 1994 Jan;11(2):315–321. doi: 10.1111/j.1365-2958.1994.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg H. F., Ackerman S. J., Tenen D. G. An alternative method for labeling oligonucleotide probes for screening cDNA libraries. Biotechniques. 1990 Apr;8(4):384–384. [PubMed] [Google Scholar]
- Sasakawa C., Yoshikawa M. A series of Tn5 variants with various drug-resistance markers and suicide vector for transposon mutagenesis. Gene. 1987;56(2-3):283–288. doi: 10.1016/0378-1119(87)90145-4. [DOI] [PubMed] [Google Scholar]
- Sobral B. W., Honeycutt R. J., Atherly A. G., McClelland M. Electrophoretic separation of the three Rhizobium meliloti replicons. J Bacteriol. 1991 Aug;173(16):5173–5180. doi: 10.1128/jb.173.16.5173-5180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. A self-transmissible, narrow-host-range endogenous plasmid of Rhodobacter sphaeroides 2.4.1: physical structure, incompatibility determinants, origin of replication, and transfer functions. J Bacteriol. 1992 Feb;174(4):1124–1134. doi: 10.1128/jb.174.4.1124-1134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Chromosome transfer in Rhodobacter sphaeroides: Hfr formation and genetic evidence for two unique circular chromosomes. J Bacteriol. 1992 Feb;174(4):1135–1145. doi: 10.1128/jb.174.4.1135-1145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J Bacteriol. 1989 Nov;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R., Gibson J. L., Bowien B., Dijkhuizen L., Meijer W. G. Uniform designation for genes of the Calvin-Benson-Bassham reductive pentose phosphate pathway of bacteria. FEMS Microbiol Lett. 1992 Dec 1;78(2-3):107–110. doi: 10.1111/j.1574-6968.1992.tb05551.x. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A., SIU P. M. BIOSYNTHESIS OF SERINE IN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Jun;85:1431–1439. doi: 10.1128/jb.85.6.1431-1439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel R., Herrmann R. Cloning of the complete Mycoplasma pneumoniae genome. Nucleic Acids Res. 1989 Sep 12;17(17):7029–7043. doi: 10.1093/nar/17.17.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel R., Herrmann R. Physical mapping of the Mycoplasma pneumoniae genome. Nucleic Acids Res. 1988 Sep 12;16(17):8323–8336. doi: 10.1093/nar/16.17.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Weisburg W. G., Paster B. J., Madigan M. T., Fowler V. J., Hahn C. M., Blanz P., Gupta R., Nealson K. H. The phylogeny of purple bacteria: the alpha subdivision. Syst Appl Microbiol. 1984;5:315–326. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]
- Zuerner R. L., Herrmann J. L., Saint Girons I. Comparison of genetic maps for two Leptospira interrogans serovars provides evidence for two chromosomes and intraspecies heterogeneity. J Bacteriol. 1993 Sep;175(17):5445–5451. doi: 10.1128/jb.175.17.5445-5451.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]