Abstract
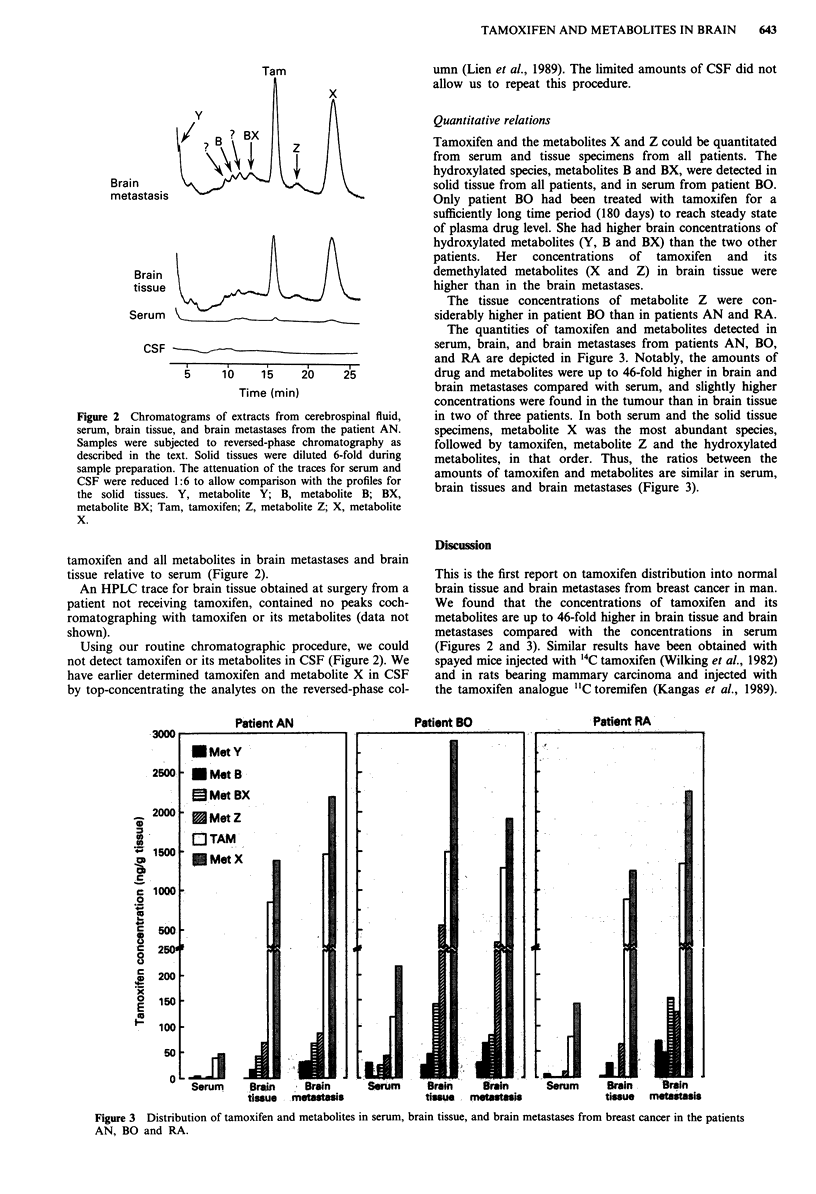
We determined the amount of tamoxifen, N-desmethyltamoxifen (metabolite X), N-desdimethyltamoxifen (metabolite Z), and hydroxylated metabolites (Y, B, BX) in brain metastases from breast cancer and in the surrounding brain tissues. Specimens were collected from the breast cancer patients who received tamoxifen for 7-180 days and with the last dose taken within 28 h before surgical removal of the tumour. The concentrations of tamoxifen and its metabolites were up to 46-fold higher in the brain metastatic tumour and brain tissue than in serum. Metabolite X was the most abundant species followed by tamoxifen and metabolite Z. Small but significant amounts of the hydroxylated metabolites, trans-1(4-beta-hydroxyethoxyphenyl)-1,2-diphenylbut-1-ene (metabolite Y), 4-hydroxytamoxifen (metabolite B) and 4-hydroxy-N-desmethyltamoxifen (metabolite BX) were detected in most specimens. The ratios between the concentrations of tamoxifen and various metabolites were similar in tumour, brain and serum. This is the first report on the distribution of tamoxifen and metabolites into human brain and brain tumour, and the data form a basis for further investigation into the therapeutic effects of tamoxifen on brain metastases from breast cancer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biswas R., Vonderhaar B. K. Antiestrogen inhibition of prolactin-induced growth of the Nb2 rat lymphoma cell line. Cancer Res. 1989 Nov 15;49(22):6295–6299. [PubMed] [Google Scholar]
- Brandes L. J., Bogdanovic R. P., Cawker M. D., Bose R. The antiproliferative properties of tamoxifen and phenothiazines may be mediated by a unique histamine receptor (?H3) distinct from the calmodulin-binding site. Cancer Chemother Pharmacol. 1986;18(1):21–23. doi: 10.1007/BF00253057. [DOI] [PubMed] [Google Scholar]
- Carey R. W., Davis J. M., Zervas N. T. Tamoxifen-induced regression of cerebral metastases in breast carcinoma. Cancer Treat Rep. 1981 Sep-Oct;65(9-10):793–795. [PubMed] [Google Scholar]
- Colomer R., Cosos D., Del Campo J. M., Boada M., Rubio D., Salvador L. Brain metastases from breast cancer may respond to endocrine therapy. Breast Cancer Res Treat. 1988 Sep;12(1):83–86. doi: 10.1007/BF01805745. [DOI] [PubMed] [Google Scholar]
- DINGELL J. V., SULSER F., GILLETTE J. R. SPECIES DIFFERENCES IN THE METABOLISM OF IMIPRAMINE AND DESMETHYLIMIPRAMINE (DMI). J Pharmacol Exp Ther. 1964 Jan;143:14–22. [PubMed] [Google Scholar]
- Dauplat J., Nieberg R. K., Hacker N. F. Central nervous system metastases in epithelial ovarian carcinoma. Cancer. 1987 Nov 15;60(10):2559–2562. doi: 10.1002/1097-0142(19871115)60:10<2559::aid-cncr2820601035>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- DiStefano A., Yong Yap Y., Hortobagyi G. N., Blumenschein G. R. The natural history of breast cancer patients with brain metastases. Cancer. 1979 Nov;44(5):1913–1918. doi: 10.1002/1097-0142(197911)44:5<1913::aid-cncr2820440554>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea J. F., Walther B., Perrin R., Minn A., Siest G. Inducibility of rat brain drug-metabolizing enzymes. Eur J Drug Metab Pharmacokinet. 1987 Oct-Dec;12(4):263–265. doi: 10.1007/BF03189910. [DOI] [PubMed] [Google Scholar]
- Ginsberg S., Kirshner J., Reich S., Panasci L., Finkelstein T., Fandrich S., Fitzpatrick A., Shechtman L., Comis R. Systemic chemotherapy for a primary germ cell tumor of the brain: a pharmacokinetic study. Cancer Treat Rep. 1981 May-Jun;65(5-6):477–483. [PubMed] [Google Scholar]
- Greig N. H. Optimizing drug delivery to brain tumors. Cancer Treat Rev. 1987 Mar;14(1):1–28. doi: 10.1016/0305-7372(87)90048-x. [DOI] [PubMed] [Google Scholar]
- Grisoli F., Vincentelli F., Foa J., Lavail G., Salamon G. Effect of bromocriptine on brain metastasis in breast cancer. Lancet. 1981 Oct 3;2(8249):745–746. doi: 10.1016/s0140-6736(81)91065-5. [DOI] [PubMed] [Google Scholar]
- Hansen S. B., Galsgård H., von Eyben F. E., Westergaard-Nielsen V., Wolf-Jensen J. Tamoxifen for brain metastases from breast cancer. Ann Neurol. 1986 Oct;20(4):544–544. doi: 10.1002/ana.410200419. [DOI] [PubMed] [Google Scholar]
- Jordan V. C. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984 Dec;36(4):245–276. [PubMed] [Google Scholar]
- Kangas L., Haaparanta M., Paul R., Roeda D., Sipilä H. Biodistribution and scintigraphy of 11C-toremifene in rats bearing DMBA-induced mammary carcinoma. Pharmacol Toxicol. 1989 Apr;64(4):373–377. doi: 10.1111/j.1600-0773.1989.tb00668.x. [DOI] [PubMed] [Google Scholar]
- LANG E. F., SLATER J. METASTATIC BRAIN TUMORS. RESULTS OF SURGICAL AND NONSURGICAL TREATMENT. Surg Clin North Am. 1964 Jun;44:865–872. doi: 10.1016/s0039-6109(16)37308-x. [DOI] [PubMed] [Google Scholar]
- Lacoursiere R. B., Spohn H. E. How long does chlorpromazine last? J Nerv Ment Dis. 1976 Oct;163(4):267–275. doi: 10.1097/00005053-197610000-00006. [DOI] [PubMed] [Google Scholar]
- Lazier C. B., Bapat B. V. Antiestrogen binding sites: general and comparative properties. J Steroid Biochem. 1988 Oct;31(4B):665–669. doi: 10.1016/0022-4731(88)90016-7. [DOI] [PubMed] [Google Scholar]
- Le Chevalier T., Smith F. P., Caille P., Constans J. P., Rouesse J. G. Sites of primary malignancies in patients presenting with cerebral metastases. A review of 120 cases. Cancer. 1985 Aug 15;56(4):880–882. doi: 10.1002/1097-0142(19850815)56:4<880::aid-cncr2820560430>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Lien E. A., Solheim E., Kvinnsland S., Ueland P. M. Identification of 4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res. 1988 Apr 15;48(8):2304–2308. [PubMed] [Google Scholar]
- Lien E. A., Solheim E., Lea O. A., Lundgren S., Kvinnsland S., Ueland P. M. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989 Apr 15;49(8):2175–2183. [PubMed] [Google Scholar]
- Lien E. A., Ueland P. M., Solheim E., Kvinnsland S. Determination of tamoxifen and four metabolites in serum by low-dispersion liquid chromatography. Clin Chem. 1987 Sep;33(9):1608–1614. [PubMed] [Google Scholar]
- O'Brian C. A., Housey G. M., Weinstein I. B. Specific and direct binding of protein kinase C to an immobilized tamoxifen analogue. Cancer Res. 1988 Jul 1;48(13):3626–3629. [PubMed] [Google Scholar]
- Pasqualini J. R., Gelly C., Nguyen B. L. Metabolism and biologic response of estrogen sulfates in hormone-dependent and hormone-independent mammary cancer cell lines. Effect of antiestrogens. Ann N Y Acad Sci. 1990;595:106–116. doi: 10.1111/j.1749-6632.1990.tb34286.x. [DOI] [PubMed] [Google Scholar]
- Robertson D. W., Katzenellenbogen J. A., Long D. J., Rorke E. A., Katzenellenbogen B. S. Tamoxifen antiestrogens. A comparison of the activity, pharmacokinetics, and metabolic activation of the cis and trans isomers of tamoxifen. J Steroid Biochem. 1982 Jan;16(1):1–13. doi: 10.1016/0022-4731(82)90137-6. [DOI] [PubMed] [Google Scholar]
- Rosner D., Nemoto T., Lane W. W. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986 Aug 15;58(4):832–839. doi: 10.1002/1097-0142(19860815)58:4<832::aid-cncr2820580404>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Sparrow G. E., Rubens R. D. Brain metastases from breast cancer: clinical course, prognosis and influence of treatment. Clin Oncol. 1981 Dec;7(4):291–301. [PubMed] [Google Scholar]
- Su H. D., Mazzei G. J., Vogler W. R., Kuo J. F. Effect of tamoxifen, a nonsteroidal antiestrogen, on phospholipid/calcium-dependent protein kinase and phosphorylation of its endogenous substrate proteins from the rat brain and ovary. Biochem Pharmacol. 1985 Oct 15;34(20):3649–3653. doi: 10.1016/0006-2952(85)90225-4. [DOI] [PubMed] [Google Scholar]
- Tang B. L., Teo C. C., Sim K. Y., Ng M. L., Kon O. L. Cytostatic effect of antiestrogens in lymphoid cells: relationship to high affinity antiestrogen-binding sites and cholesterol. Biochim Biophys Acta. 1989 Nov 20;1014(2):162–172. doi: 10.1016/0167-4889(89)90029-3. [DOI] [PubMed] [Google Scholar]
- Tsukada Y., Fouad A., Pickren J. W., Lane W. W. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983 Dec 15;52(12):2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Vlasveld L. T., Beynen J. H., Boogerd W., Ten Bokkel Huinink W. W., Rodenhuis S. Complete remission of brain metastases of ovarian cancer following high-dose carboplatin: a case report and pharmacokinetic study. Cancer Chemother Pharmacol. 1990;25(5):382–383. doi: 10.1007/BF00686244. [DOI] [PubMed] [Google Scholar]
- Weiss D. J., Gurpide E. Non-genomic effects of estrogens and antiestrogens. J Steroid Biochem. 1988 Oct;31(4B):671–676. doi: 10.1016/0022-4731(88)90017-9. [DOI] [PubMed] [Google Scholar]
- Wilking N., Appelgren L. E., Carlström K., Pousette A., Theve N. O. The distribution and metabolism of 14C-labelled tamoxifen in spayed female mice. Acta Pharmacol Toxicol (Copenh) 1982 Mar;50(3):161–168. doi: 10.1111/j.1600-0773.1982.tb00958.x. [DOI] [PubMed] [Google Scholar]
- Zimm S., Wampler G. L., Stablein D., Hazra T., Young H. F. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981 Jul 15;48(2):384–394. doi: 10.1002/1097-0142(19810715)48:2<384::aid-cncr2820480227>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]


