Abstract
The invasiveness of MCF-7 human mammary carcinoma cells was tested in vitro via confronting cultures with embryonic chick heart fragments. Invasive (e.g. MCF-7/6) and non-invasive (e.g. MCF-7/AZ) variants were detected. Automated image analysis of time-lapse video-microscopy recordings showed that the plasma membrane ruffling activity of the invasive MCF-7/6 variant was higher than the ruffling activity of the non-invasive MCF-7/AZ variant. Addition of all-trans-retinoic acid to the culture medium (10(-6) M) inhibited both invasion and ruffling of MCF-7/6 cells, while MCF-7/AZ cells became invasive and acquired an increased ruffling by the same type of treatment. A similar opposite effect on MCF-7 cells was not found after treatment with other ligands of the nuclear steroid/thyroid receptor superfamily. Triiodo-l-thyronine (up to 10(-5) M) and beta-oestradiol (up to 10(-6) M) did not alter the invasiveness of the cells, while dexamethasone (10(-6) M) and the pure anti-oestrogen ICI 164,384 inhibited both invasion and ruffling. Our data show that retinoic acid can modulate invasiveness in opposite directions.
Full text
PDF

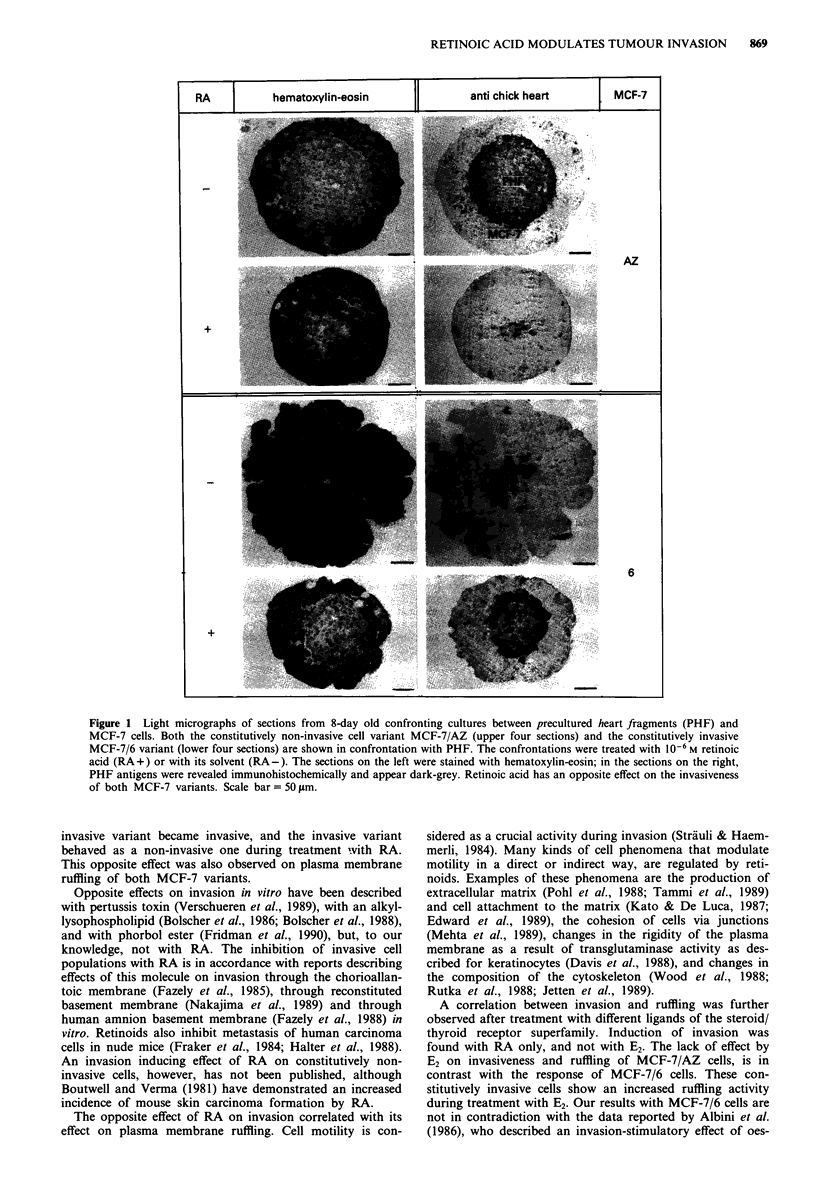
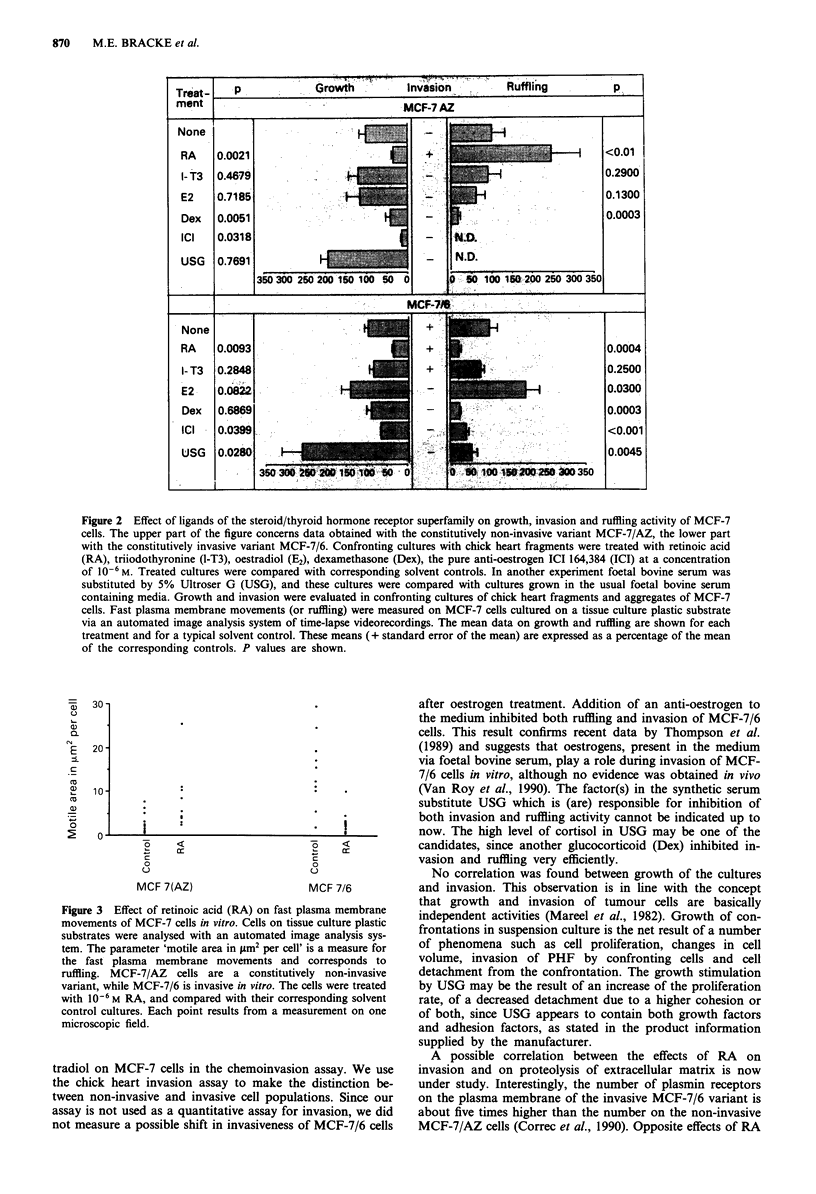


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Graf J., Kitten G. T., Kleinman H. K., Martin G. R., Veillette A., Lippman M. E. 17 beta-estradiol regulates and v-Ha-ras transfection constitutively enhances MCF7 breast cancer cell interactions with basement membrane. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8182–8186. doi: 10.1073/pnas.83.21.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M. A., Weiss D. W. Immunology of spontaneous mammary carcinomas in mice. V. Acquired tumor resistance and enhancement in strain A mice infected with mammary tumor virus. Cancer Res. 1966 Aug;26(8):1787–1800. [PubMed] [Google Scholar]
- Barnes D., Sato G. Growth of a human mammary tumour cell line in a serum-free medium. Nature. 1979 Oct 4;281(5730):388–389. doi: 10.1038/281388a0. [DOI] [PubMed] [Google Scholar]
- Bolscher J. G., Schallier D. C., Smets L. A., van Rooy H., Collard J. G., Bruyneel E. A., Mareel M. M. Effect of cancer-related and drug-induced alterations in surface carbohydrates on the invasive capacity of mouse and rat cells. Cancer Res. 1986 Aug;46(8):4080–4086. [PubMed] [Google Scholar]
- Bolscher J. G., Schallier D. C., van Rooy H., Storme G. A., Smets L. A. Modification of cell surface carbohydrates and invasive behavior by an alkyl lysophospholipid. Cancer Res. 1988 Feb 15;48(4):977–982. [PubMed] [Google Scholar]
- Bracke M. E., Van Cauwenberge R. M., Mareel M. M. (+)-Catechin inhibits the invasion of malignant fibrosarcoma cells into chick heart in vitro. Clin Exp Metastasis. 1984 Apr-Jun;2(2):161–170. doi: 10.1007/BF00052416. [DOI] [PubMed] [Google Scholar]
- Correc P., Fondanèche M. C., Bracke M., Burtin P. The presence of plasmin receptors on three mammary carcinoma MCF-7 sublines. Int J Cancer. 1990 Oct 15;46(4):745–750. doi: 10.1002/ijc.2910460432. [DOI] [PubMed] [Google Scholar]
- Edward M., Gold J. A., MacKie R. M. Modulation of melanoma cell adhesion to basement membrane components by retinoic acid. J Cell Sci. 1989 May;93(Pt 1):155–161. doi: 10.1242/jcs.93.1.155. [DOI] [PubMed] [Google Scholar]
- Fazely F., Ledinko N., Smith D. J. Inhibition by retinoids of in vitro invasive ability of human lung carcinoma cells. Anticancer Res. 1988 Nov-Dec;8(6):1387–1391. [PubMed] [Google Scholar]
- Fazely F., Moses D. C., Ledinko N. Effects of retinoids on invasion of organ cultures of chick chorioallantoic membrane by adenovirus transformed cells. In Vitro Cell Dev Biol. 1985 Jul;21(7):409–414. doi: 10.1007/BF02623472. [DOI] [PubMed] [Google Scholar]
- Fraker L. D., Halter S. A., Forbes J. T. Growth inhibition by retinol of a human breast carcinoma cell line in vitro and in athymic mice. Cancer Res. 1984 Dec;44(12 Pt 1):5757–5763. [PubMed] [Google Scholar]
- Fridman R., Lacal J. C., Reich R., Bonfil D. R., Ahn C. H. Differential effects of phorbol ester on the in vitro invasiveness of malignant and non-malignant human fibroblast cells. J Cell Physiol. 1990 Jan;142(1):55–60. doi: 10.1002/jcp.1041420108. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Halter S. A., Fraker L. D., Adcock D., Vick S. Effect of retinoids on xenotransplanted human mammary carcinoma cells in athymic mice. Cancer Res. 1988 Jul 1;48(13):3733–3736. [PubMed] [Google Scholar]
- Hendrix M. J., Wood W. R., Seftor E. A., Lotan D., Nakajima M., Misiorowski R. L., Seftor R. E., Stetler-Stevenson W. G., Bevacqua S. J., Liotta L. A. Retinoic acid inhibition of human melanoma cell invasion through a reconstituted basement membrane and its relation to decreases in the expression of proteolytic enzymes and motility factor receptor. Cancer Res. 1990 Jul 1;50(13):4121–4130. [PubMed] [Google Scholar]
- Jetten A. M., George M. A., Smits H. L., Vollberg T. M. Keratin 13 expression is linked to squamous differentiation in rabbit tracheal epithelial cells and down-regulated by retinoic acid. Exp Cell Res. 1989 Jun;182(2):622–634. doi: 10.1016/0014-4827(89)90264-4. [DOI] [PubMed] [Google Scholar]
- Kato S., De Luca L. M. Retinoic acid modulates attachment of mouse fibroblasts to laminin substrates. Exp Cell Res. 1987 Dec;173(2):450–462. doi: 10.1016/0014-4827(87)90285-0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Mareel M. M., De Bruyne G. K., Vandesande F., Dragonetti C. Immunohistochemical study of embryonic chick heart invaded by malignant cells in three-dimensional culture. Invasion Metastasis. 1981;1(3):195–204. [PubMed] [Google Scholar]
- Mareel M. M., Van Roy F. M., Messiaen L. M., Boghaert E. R., Bruyneel E. A. Qualitative and quantitative analysis of tumour invasion in vivo and in vitro. J Cell Sci Suppl. 1987;8:141–163. doi: 10.1242/jcs.1987.supplement_8.8. [DOI] [PubMed] [Google Scholar]
- Mareel M., Kint J., Meyvisch C. Methods of study of the invasion of malignant C3H-mouse fibroblasts into embryonic chick heart in vitro. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979 May 4;30(1):95–111. doi: 10.1007/BF02889094. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Bertram J. S., Loewenstein W. R. The actions of retinoids on cellular growth correlate with their actions on gap junctional communication. J Cell Biol. 1989 Mar;108(3):1053–1065. doi: 10.1083/jcb.108.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Lotan D., Baig M. M., Carralero R. M., Wood W. R., Hendrix M. J., Lotan R. Inhibition by retinoic acid of type IV collagenolysis and invasion through reconstituted basement membrane by metastatic rat mammary adenocarcinoma cells. Cancer Res. 1989 Apr 1;49(7):1698–1706. [PubMed] [Google Scholar]
- Neuman T., Stephens R. W., Salonen E. M., Timmusk T., Vaheri A. Induction of morphological differentiation of human neuroblastoma cells is accompanied by induction of tissue-type plasminogen activator. J Neurosci Res. 1989 Jul;23(3):274–281. doi: 10.1002/jnr.490230305. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Pohl J., Radler-Pohl A., Schirrmacher V. A model to account for the effects of oncogenes, TPA, and retinoic acid on the regulation of genes involved in metastasis. Cancer Metastasis Rev. 1988 Dec;7(4):347–356. doi: 10.1007/BF00051375. [DOI] [PubMed] [Google Scholar]
- Ponglikitmongkol M., Green S., Chambon P. Genomic organization of the human oestrogen receptor gene. EMBO J. 1988 Nov;7(11):3385–3388. doi: 10.1002/j.1460-2075.1988.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicoff M., Medrano E. E., Podhajcer O. L., Bravo A. I., Bover L., Mordoh J. Subpopulations of MCF7 cells separated by Percoll gradient centrifugation: a model to analyze the heterogeneity of human breast cancer. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7295–7299. doi: 10.1073/pnas.84.20.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. Retinoic acid receptor. Towards a biochemistry of morphogenesis. Nature. 1987 Dec 3;330(6147):420–421. doi: 10.1038/330420a0. [DOI] [PubMed] [Google Scholar]
- Rutka J. T., De Armond S. J., Giblin J., McCulloch J. R., Wilson C. B., Rosenblum M. L. Effect of retinoids on the proliferation, morphology and expression of glial fibrillary acidic protein of an anaplastic astrocytoma cell line. Int J Cancer. 1988 Sep 15;42(3):419–427. doi: 10.1002/ijc.2910420319. [DOI] [PubMed] [Google Scholar]
- Seibert K., Shafie S. M., Triche T. J., Whang-Peng J. J., O'Brien S. J., Toney J. H., Huff K. K., Lippman M. E. Clonal variation of MCF-7 breast cancer cells in vitro and in athymic nude mice. Cancer Res. 1983 May;43(5):2223–2239. [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Sträuli P., Haemmerli G. The role of cancer cell motility in invasion. Cancer Metastasis Rev. 1984;3(2):127–141. doi: 10.1007/BF00047660. [DOI] [PubMed] [Google Scholar]
- Tammi R., Ripellino J. A., Margolis R. U., Maibach H. I., Tammi M. Hyaluronate accumulation in human epidermis treated with retinoic acid in skin organ culture. J Invest Dermatol. 1989 Mar;92(3):326–332. doi: 10.1111/1523-1747.ep12277125. [DOI] [PubMed] [Google Scholar]
- Thompson E. W., Katz D., Shima T. B., Wakeling A. E., Lippman M. E., Dickson R. B. ICI 164,384, a pure antagonist of estrogen-stimulated MCF-7 cell proliferation and invasiveness. Cancer Res. 1989 Dec 15;49(24 Pt 1):6929–6934. [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Van Roy F., Mareel M., Vleminckx K., Beyaert R., Fiers W., Devleeschouwer N., Muquardt C., Legros N., Bracke M., Leclercq G. Hormone sensitivity in vitro and in vivo of v-ras-transfected MCF-7 cell derivatives. Int J Cancer. 1990 Sep 15;46(3):522–532. doi: 10.1002/ijc.2910460332. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., Coene M. C., Goossens J., Cools W., Monbaliu J. Effects of cytochrome P-450 inhibitors on the in vivo metabolism of all-trans-retinoic acid in rats. J Pharmacol Exp Ther. 1990 Jan;252(1):365–369. [PubMed] [Google Scholar]
- Verschueren H., Van Hecke D., Hannecart-Pokorni E., Dekegel D., De Baetselier P. Dual effects of pertussis toxin on in vitro invasive behavior of metastatic lymphoma variants. Clin Exp Metastasis. 1989 Sep-Oct;7(5):541–555. doi: 10.1007/BF01753814. [DOI] [PubMed] [Google Scholar]
- Vic P., Vignon F., Derocq D., Rochefort H. Effect of estradiol on the ultrastructure of the MCF7 human breast cancer cells in culture. Cancer Res. 1982 Feb;42(2):667–673. [PubMed] [Google Scholar]
- Wood E. J., Raxworthy M. J., Holland D. B. Retinoids and the epidermis. Biochem Soc Trans. 1988 Oct;16(5):668–671. doi: 10.1042/bst0160668. [DOI] [PubMed] [Google Scholar]



