Abstract
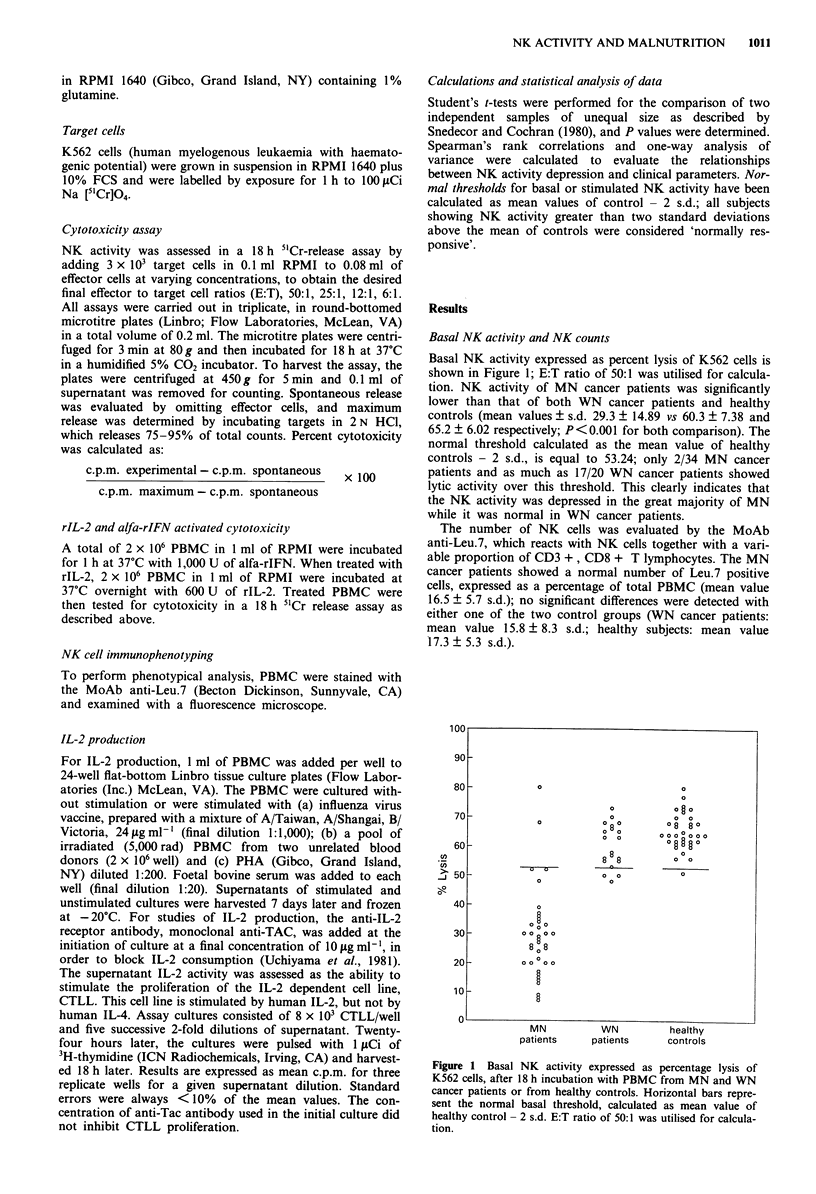
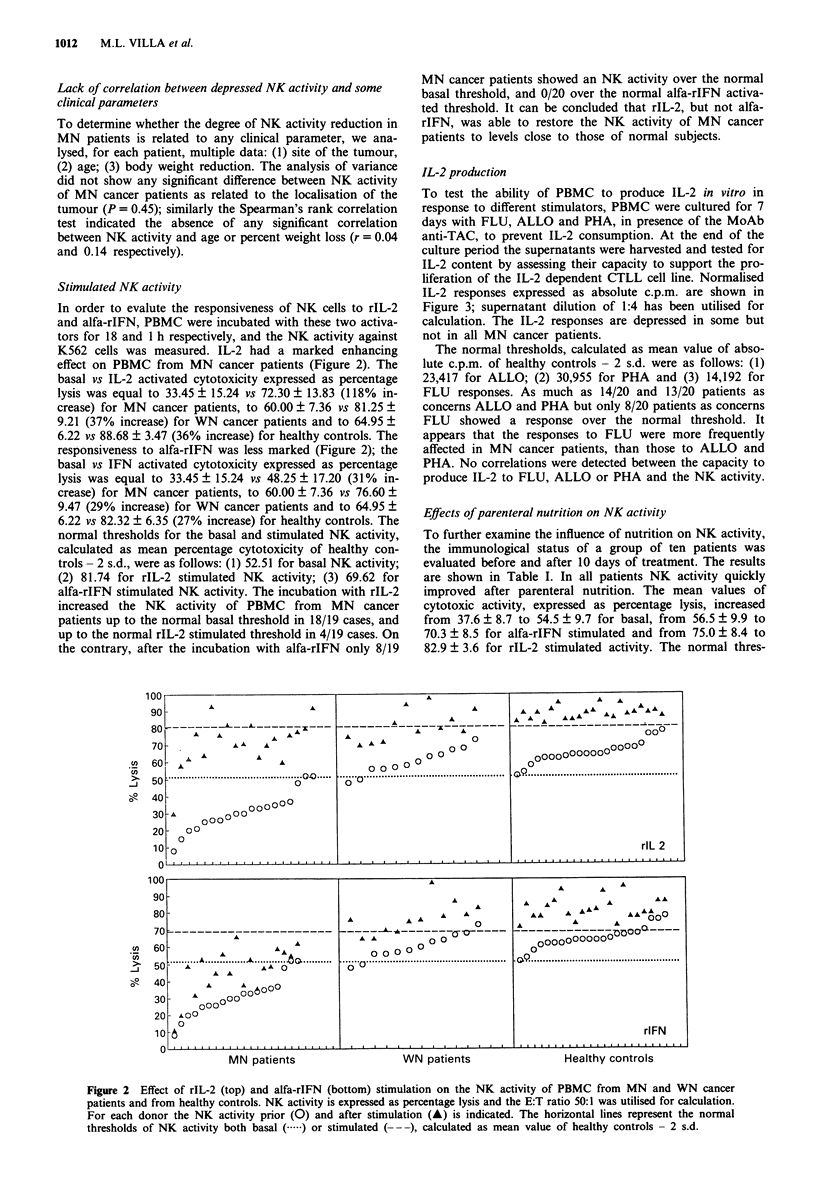
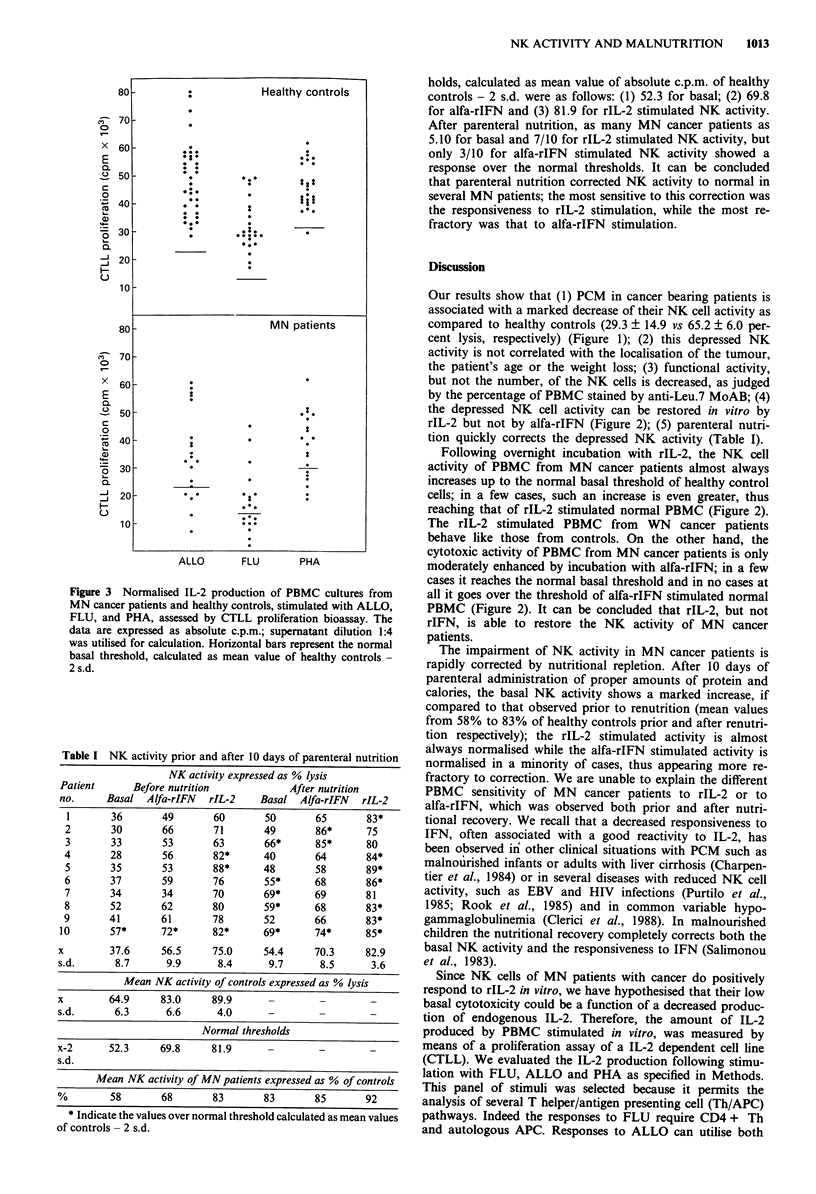
Natural killer (NK) cell activity was measured in the peripheral blood mononuclear cells (PBMC) from malnourished (MN) and well-nourished (WN) cancer patients and in healthy controls. A marked depression of NK activity was observed in MN cancer patients with moderate protein-calorie malnutrition (PCM), but not in WN cancer patients nor in the healthy controls. The depression of NK activity did not correlate with the localisation of the tumour, patient's age or body weight reduction. The defective NK activity of PBMC from MN cancer patients was restored to normal by rIL-2, but not by alfa-rIFN. Parenteral nutrition of MN patients with the proper amount of proteins and calories quickly corrected the depressed NK activity, indicating a central role of malnutrition in the genesis of their immune disfunction. PBMC from MN cancer patients produced lower amounts of IL-2, as compared with healthy controls, when stimulated in vitro; the most frequently affected were the responses to recall antigens such as influenza virus vaccine (FLU), while those to allogeneic PBMC (ALLO) and phytohaemagglutinin (PHA) were less affected. However, for each patient the ability to produce IL-2 in vitro did not correlate with NK activity, thus showing how the impairment of NK activity is not subsequent to a decreased production of endogenous IL-2. In summary, it can be concluded that malnutrition, rather than malignancy, plays a major role in the immune dysfunction of cancer patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B. The presence of cachectin/tumor necrosis factor in human disease states. Am J Med. 1988 Sep;85(3):287–288. doi: 10.1016/0002-9343(88)90575-x. [DOI] [PubMed] [Google Scholar]
- Bozzetti F. Effects of artificial nutrition on the nutritional status of cancer patients. JPEN J Parenter Enteral Nutr. 1989 Jul-Aug;13(4):406–420. doi: 10.1177/0148607189013004406. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Nutritional regulation of immunity and risk of infection in old age. Immunology. 1989 Jun;67(2):141–147. [PMC free article] [PubMed] [Google Scholar]
- Charpentier B., Franco D., Paci L., Charra M., Martin B., Vuitton D., Fries D. Deficient natural killer cell activity in alcoholic cirrhosis. Clin Exp Immunol. 1984 Oct;58(1):107–115. [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Villa M. L., Mantovani M., Rugarli C. NK cell activity and monocyte dysfunctions in a patient with common variable hypogammaglobulinemia. J Clin Lab Immunol. 1988 Nov;27(3):143–147. [PubMed] [Google Scholar]
- Gordon C., Wofsy D. Effects of recombinant murine tumor necrosis factor-alpha on immune function. J Immunol. 1990 Mar 1;144(5):1753–1758. [PubMed] [Google Scholar]
- Gross R. L., Newberne P. M. Role of nutrition in immunologic function. Physiol Rev. 1980 Jan;60(1):188–302. doi: 10.1152/physrev.1980.60.1.188. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Douglas S. D., Braden K., Geller S. A. Antibacterial functions of macrophages in experimental protein-calorie malnutrition. I. Description of the model, morphologic observations, and macrophage surface IgG receptors. J Infect Dis. 1978 Aug;138(2):125–133. doi: 10.1093/infdis/138.2.125. [DOI] [PubMed] [Google Scholar]
- Law D. K., Dudrick S. J., Abdou N. I. Immunocompetence of patients with protein-calorie malnutrition. The effects of nutritional repletion. Ann Intern Med. 1973 Oct;79(4):545–550. doi: 10.7326/0003-4819-79-4-545. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Altman L. C., Alvares O. F. The effects of severe protein-calorie malnutrition on antibacterial defense mechanisms in the rat lung. Am Rev Respir Dis. 1983 Dec;128(6):1013–1019. doi: 10.1164/arrd.1983.128.6.1013. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C. R., Oomura Y., Kai Y. Tumor necrosis factor and interleukin-1 beta: suppression of food intake by direct action in the central nervous system. Brain Res. 1988 May 10;448(1):106–114. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Tatsumi E., Manolov G., Manolova Y., Harada S., Lipscomb H., Krueger G. Epstein-Barr virus as an etiological agent in the pathogenesis of lymphoproliferative and aproliferative diseases in immune deficient patients. Int Rev Exp Pathol. 1985;27:113–183. [PubMed] [Google Scholar]
- Rook A. H., Hooks J. J., Quinnan G. V., Lane H. C., Manischewitz J. F., Macher A. M., Masur H., Fauci A. S., Djeu J. Y. Interleukin 2 enhances the natural killer cell activity of acquired immunodeficiency syndrome patients through a gamma-interferon-independent mechanism. J Immunol. 1985 Mar;134(3):1503–1507. [PubMed] [Google Scholar]
- Salimonu L. S., Ojo-Amaize E., Johnson A. O., Laditan A. A., Akinwolere O. A., Wigzell H. Depressed natural killer cell activity in children with protein--calorie malnutrition. II. Correction of the impaired activity after nutritional recovery. Cell Immunol. 1983 Nov;82(1):210–215. doi: 10.1016/0008-8749(83)90154-5. [DOI] [PubMed] [Google Scholar]
- Schattner A., Steinbock M., Tepper R., Schonfeld A., Vaisman N., Hahn T. Tumour necrosis factor production and cell-mediated immunity in anorexia nervosa. Clin Exp Immunol. 1990 Jan;79(1):62–66. doi: 10.1111/j.1365-2249.1990.tb05127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman W. E., Sleisenger M., Eriksson E., Koo G. C. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J Immunol. 1987 Jun 15;138(12):4539–4544. [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Vaisman N., Schattner A., Hahn T. Tumor necrosis factor production during starvation. Am J Med. 1989 Jul;87(1):115–115. doi: 10.1016/s0002-9343(89)80497-8. [DOI] [PubMed] [Google Scholar]


