Abstract
Extensive glycosylation and variable loops of the HIV envelope protein (Env) are reported to shield some neutralizing epitopes. Here, we investigated the immunogenicity of mutated HIV Envs presented in virus-like particles (VLPs). We immunized mice with simian human immunodeficiency virus (SHIV) VLPs containing mutant HIV Env with reduced glycosylation (3G), variable loop-deleted mutations (dV1V2), or combinations of both types of mutations (3G-dV2−1G), and evaluated immune responses. Immune sera from mice that received VLPs with modified HIV Envs (3G or dV1V2) showed higher neutralizing activities against the homologous HIV 89.6 virus as well as heterologous viruses when compared with wild type SHIV VLP-immunized mice. Lymphocytes from immunized mice produced HIV Env-specific cytokines, with the 3G-dV2−1G mutant producing high levels of cytokines. Interestingly, both dendritic cells and B cells were found to interact with VLPs suggesting that VLPs are effective immunogens. Therefore, this study suggests that VLPs containing modified HIV Env have the potential to be developed as candidate vaccines capable of inducing cellular and humoral immune responses including neutralizing activities.
Keywords: HIV-1, virus-like particles, modification, immunogenicity
1. Introduction
Within the last decade, several vaccine approaches have been shown to be promising for induction of strong cellular immune responses controlling simian HIV (SHIV) infection and replication upon subsequent pathogenic virus challenge. Among those the most effective immunogens are live attenuated viruses, recombinant viral vectors, DNA vaccines, or combinations of these components. However, some of these vaccine components may have serious potential safety concerns such as induction of chromosomal rearrangements in the host cell and a risk to immunodeficient recipients [1, 2], which will be possible limitations on their approval for use in humans.
When the gag and env genes of HIV or simian immunodeficiency virus (SIV) are co-expressed in cells, these proteins are able to assemble on the plasma membrane to form virus-like particles (VLPs) containing viral Gag and Env proteins. The self-assembled macrostructure of VLPs presents conformational epitopes to the immune system, which are comparable to those of live virions [3, 4]. In addition, the non-infectious nature of VLPs and their lack of viral genomic material eliminate some safety concerns compared to live vector or attenuated strain based vaccines for broad and repeated application, particularly for the elderly, infant, and immunodeficient populations. Reflecting these attractive features of VLPs as vaccine candidates, several studies have focused on developing viral vaccines based on VLPs including hepatitis virus, papillomavirus, Norwalk virus, rotavirus, parvovirus, and influenza virus [5-13].
One of the major obstacles in developing an effective AIDS vaccine is the difficulty in inducing neutralizing antibodies that are broadly reactive against many HIV-1 isolates. Several properties of HIV Env contribute to this difficulty. HIV-1 Env, the main neutralization target, is highly variable among isolates with five hyper-variable regions (V1-V5) which induce strain specific antibodies [14]. HIV Env is also extensively glycosylated, with 23 to 27 N-linked glycosylation sites which equals half of the total protein mass [15]. Extensive glycosylations and highly variable loops may enable HIV-1 to evade host immune recognition by shielding some conserved neutralizing epitopes, such as regions of HIV-1 Env that bind to cellular receptors and coreceptors [16].
In a recent study, we demonstrated enhanced binding of broadly neutralizing monoclonal antibodies to mutated HIV-1 Envs expressed on cell surfaces [17]. The representative mutants of HIV Env with higher binding capability contained glycosylation site modifications around the receptor binding domain (3G), V1-V2 variable loop deletions (dV1V2), and a combination of both types of mutations (3G-dV2−1G). In the present study, we investigated the immunogenicity of these mutant HIV Envs when presented on VLPs, including induction of neutralizing antibodies against homologous and heterologous strains. The possible mechanism for inducing strong humoral and cellular immune responses after immunization with VLPs in the absence of adjuvant is discussed.
2. Materials and Methods
2.1 Cells, proteins, and antibodies
Spodoptera frugiperda Sf9 cells were maintained in suspension in serum-free SF900 II medium (GIBCO-BRL) in spinner flasks at a speed of 70−80 rpm. HIV 89.6 Env protein was purified from CV-1 cell (African green monkey kidney fibroblast cells) lysates infected with a recombinant vaccinia virus expressing HIV 89.6 Env (rVV-HIV 89.6) using a lectin column as described previously [18, 19]. Purified mouse IgG, IgG1, IgG2a, IgG2b, IgG3, IgA, and goat antimouse-HRP for ELISA were purchased from Southern Biotechnology Associates (Birmingham, AL).
2.2 Production of SHIV VLPs
The construction and characterization of recombinant baculoviruses (rBV) expressing mutant Envs were previously described [17]. To produce SHIV VLPs, Sf9 insect cells were coinfected with rBVs expressing the SIV Gag and mutant HIV Env at an MOI of 2 and 5, respectively. SHIV VLPs in culture supernatants were harvested and purified by sucrose gradient ultracentrifugation as described [20, 21]. Incorporation of HIV Envs into VLPs was confirmed by Western blot using monkey anti-SHIV 89.6 sera (Dr. Patricia Fultz, University of Alabama at Birmingham). To determine the amount of HIV Env incorporated into VLPs, VLPs were lysed by RIPA buffer (0.1 % NP40, 0.5 % deoxycholic acid, 0.1 % SDS, 150 mM NaCL, and 50mM Tris, pH 8), serially diluted, and added to the ELISA plates coated with purified sheep antibody specific to the C5 domain of HIV (BH-10) Env (5μg/ml) (CLINIQA, Fallbrook, CA). Purified HIV-1 gp120 protein (NIH AIDS reagent program) was used as a standard. The amount of HIV Env captured onto the ELISA plate was estimated using pooled HIV patient sera (NIH AIDS reagent program). The total protein concentration of VLPs was determined by the detergent compatible Bio-Rad protein assay kit.
2.3 Immunizations, blood sample collection, and ELISA
Groups of female inbred BALB/c mice (Charles River) aged 6 to 8 weeks were used. Individual mice were immunized subcutaneously with 50 μg VLP in 100 μl of PBS. Blood samples were collected by retro-orbital plexus puncture before immunization and 2 weeks after every immunization. After clotting and centrifugation, serum samples were collected and stored at −20 °C prior to antibody titration.
All sera were individually collected, and IgG, IgG1, IgG2a, IgG2b, IgG3, and IgA antibody titers to HIV Env were determined by enzyme-linked immunosorbent assay (ELISA) as described [19]. Briefly, 96-well microtiter plates (Nunc-Immuno Plate MaxiSorp™) were coated with 100 μl/well of purified HIV 89.6 Env protein (4 μg/ml) or the V3 loop peptide (amino acids 309 to 318, IGPGRAFYAR 4 μg/ml) in coating buffer (0.1 M Sodium carbonate, pH 9.5) at 4 °C overnight. After blocking and wash, the plates were incubated with horseradish peroxidase (HRP) -labeled goat anti-mouse antibodies (Southern Biotechnology, Birmingham, AL) at 37 °C for 1.5 hrs. After washing, the substrate O-phenylenediamine (OPD) (Zymed, San Francisco, CA) in citrate-phosphate buffer (pH 5.0) containing 0.03% H2O2 (Sigma) was used to develop color, and optical density at 450 nm was read by ELISA reader (Model 680, Bio-Rad).
2.4 ELISPOT and cytokine ELISA
Spleens were collected from individual mice at 2 weeks after the final immunization, a single cell suspension was prepared, and used for enzyme-linked immunospot (ELISPOT) and cytokine ELISA as described [19]. Briefly, all antibodies against mouse cytokines used in ELISPOT assays were purchased (BD-PharMingen, San Diego, Calif.). Anti-mouse IFN-γ, IL-2, IL-4 and IL-5 antibodies (3 μg/ml in coating buffer) were used to coat Multiscreen 96-well filtration plates (Millipore) at 4 °C overnight and freshly isolated splenocytes (1.5 × 106 cells) were added to each well. H2-Dd restricted HIV 89.6 Env peptide, IGPGRAFYAR [22] or wild type SHIV VLPs were added at a concentration of 2 μg/ml, and plates were incubated for 36 h at 37 °C with 5% CO2. Biotinylated anti-mouse cytokine (IFN-γ, IL-2, IL-4 and IL-5) antibodies (1.5 μg/ml), streptavidin-HRP (BD-PharMingen, San Jose, CA), and stable diaminobenzidine (Invitrogen, Carlsbad, CA) were used to develop spots as described previously [19]. Spots were counted by an ImmunoSpot ELISPOT reader (Cellular Technology, Ltd.). Cytokine ELISA was performed as described previously [23]. Briefly, spleen lymphocytes (1.5 × 106 cells/well) were cultured in the presence of 5 μg of peptide, VLPs, or concanavalin A (5 μg/ml) as described above. The culture supernatants were harvested on day 3 after incubation at 37 °C, and cytokine levels (IFN-γ, TNF-α, IL-4, IL-6 and IL-10) were determined. OptEIA™ Set Mouse IFN-γ and IL-4 (BD-PharMingen, San Jose, CA), and Ready-Set-Go TNF-α, IL-6, IL-10 (eBioscience, San Diego, CA) were used for detecting cytokine levels in the supernatants following the manufacture’s procedures.
2.5 Neutralization assay
Neutralization assays were performed as described previously [19, 24]. JC 53 BL cells were used with HIV-1 89.6, IIIB or pseudovirions of a non-T-cell line primary isolate YU2. In brief, serum samples were heat inactivated (56 °C, 30 min), serially two-fold diluted in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS, and a final volume of 30 μl was mixed with 30 μl of diluted virus stock containing approximately 50 to 100 infectious particles for 1 hr at 37 °C prior to infection. At 3 days post infection, the medium was removed, and the cells were fixed and stained. The blue cells in the wells without antisera indicated the total number of infectious virus particles. The neutralization activity was expressed as the percentage reduction (%) of infectious spots compared to that of the well without serum sample.
2.6 Biotinylation of VLPs and FACS
To conjugate VLPs, 300 μg of VLPs were mixed with 60 μg of biotin and incubated in the dark for 60 min at room temperature. Tris-buffered saline (10 mM Tris pH 7.4, 150 mM NaCl) was added to quench the remaining free biotin. The biotin conjugated VLPs were washed with Tris buffer twice by ultracentrifugation through a 20 % sucrose cushion and followed by dialysis against PBS buffer. The protein concentration of VLPs was determined by a detergent compatible protein assay kit (Bio-Rad) and levels of biotin were determined with HRP-streptavidin by ELISA. All VLPs were found to be conjugated with biotin at similar efficiency (data not shown).
For fluorescence-activated cell sorting (FACS), 1μg of biotin labeled VLPs were mixed with naïve mousespleen cells or dendritic cell (DC) enriched splenocytes (1×106 cells). DC-expanded splenocytes were prepared by injection of Flt3 ligand encoding DNA as described [25]. The mixture of VLPs and cells was incubated for 20 min at 4 °C, washed with PBS containing 2% bovine fetal serum, and stained with APC or PE streptavidin and phenotypic cell surface antibodies (PE conjugated CD11c, CD19, FITC conjugated CD4 and MHCII, and PerCP conjugated CD8, and Fc blockers CD16 and CD32 antibodies). 50,000 events were collected and analyzed with a FACS Calibur instrument (Becton Dickinson) and WINMDI 2.8 software (The Scripps Research Institute Cytometry Software).
2.7 Statistical analysis
Serum antibody levels were recorded for individuals within all groups. Statistical comparisons of these data were carried out using the ANOVA and Npar1-way Kruskal-Wallis tests of the PC-SAS system. A value of P < 0.05 was considered significant. Immunization experiments were repeated to confirm that immune responses were reproducible.
3. Results
3.1 Production of SHIV VLPs with mutated Env.
In a recent study [17], we described the antigenicity of a series of mutant HIV Env proteins expressed on the cell surface using broadly neutralizing monoclonal antibodies. An N-linked glycosylation motif modification which contained mutations of Asn to Gln at aa 304, 341, and 363 in the V3/C3 domains of HIV-1 gp120 CD4 binding site (3G) was found to bind to a panel of broadly neutralizing monoclonal antibodies at higher levels as compared to the wild type control. Mutants with a deletion of both the V1 and V2 loop domains (dV1V2) or with a combination of both types of mutations (3G-dV2−1G: 3G and V2 loop deletion plus a mutation of Asn to Gln at aa 635 in gp41) also bound to the neutralizing monoclonal antibodies at high levels [17].
In the present study, we investigated the immunogenicity of these mutant Envs presented in a VLP form. To produce VLPs containing mutated HIV-1 Envs, Sf9 insect cells were co-infected with rBVs expressing either HIV-1 wild type or mutant env genes (dV1V2, 3G, 3G-dV2−1G) [17] and an rBV expressing SIV Gag [26], and VLPs in the culture supernatants were collected and purified. The presence of wild type or mutant HIV Envs and SIV Gag in the released VLPs was analyzed by Western blot and all HIV Envs were found to be incorporated at similar levels (data not shown). The amounts of HIV Envs incorporated into VLPs were quantitated by an ELISA. HIV Env mutants were found to be incorporated into VLPs at equivalent levels as wild type (Fig. 1).
Fig. 1.
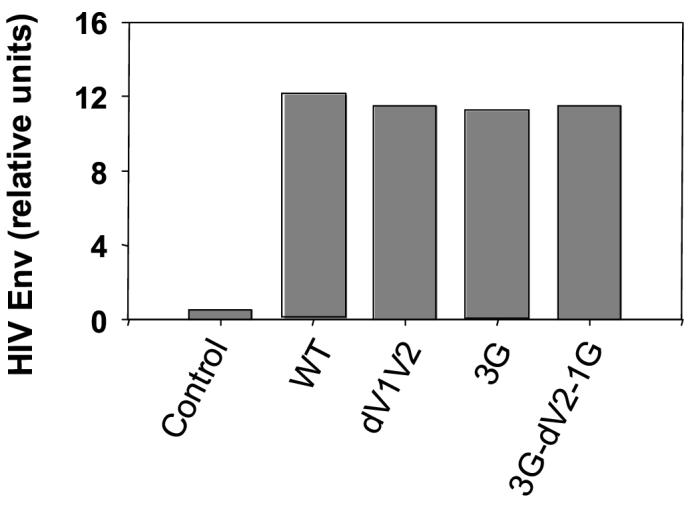
Incorporation of wild type and mutant HIV Env into VLPs. Quantitative estimation of Env in VLPs was performed using an ELISA. Gag: HIV Env-negative SIV Gag VLPs, WT: SHIV VLPs with wild type HIV Env 89.6, dV1V2: SHIV VLPs with dV1V2 mutant HIV Env, 3G: SHIV VLPs with 3G mutant HIV Env, 3G-dV2−1G: SHIV VLPs with 3G-dV2−1G mutant HIV Env.
3.2 Antibody responses after immunization with VLPs containing HIV Env mutants.
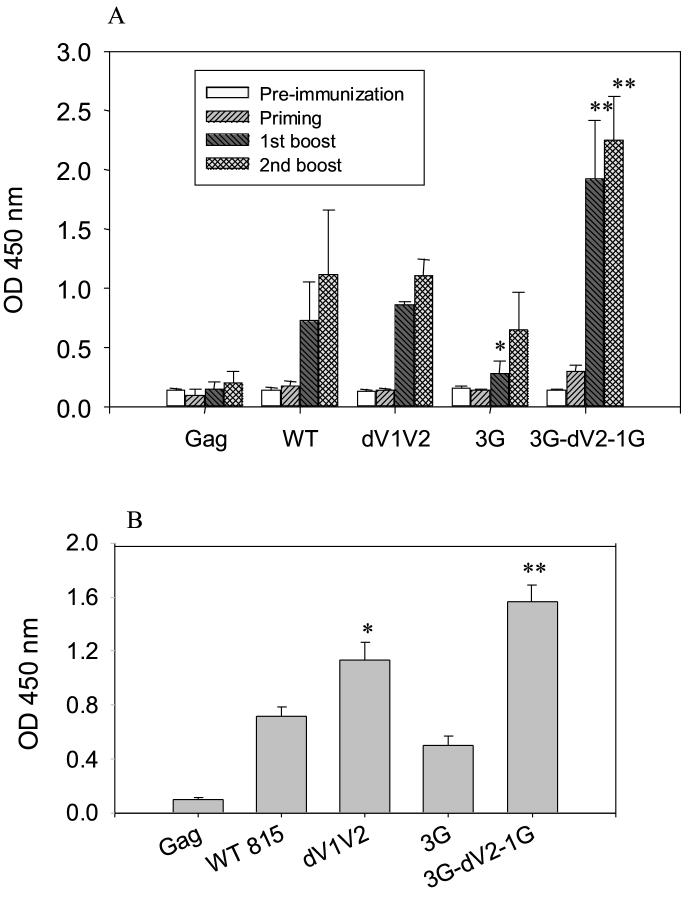
Serum samples were collected after each immunization, and HIV Env-specific total IgG antibody levels were determined by ELISA using lectin-affinity column purified HIV Env 89.6 as a coating antigen (Fig. 2A). The levels of HIV Env-specific IgG were significantly increased after the first boost (P < 0.0001) and highest after the second boost in all groups. Mice immunized with SHIV VLPs containing a combination of glycosylation and variable loop deletion (3G-dV2−1G) showed the highest level of IgG binding to HIV Env. IgG levels induced in the group of mice immunized with SHIV VLPs containing the mutant dV1V2 were similar to those induced in wild type SHIV VLPs. Antibody levels induced by the 3G mutant VLPs were lower than the wild type control after the 1st boost (p <0.05) but differences were not statistically significant after the 2nd boost. The group of mice immunized with the Env-negative SIV Gag VLPs showed only background levels of antibody responses as pre-immune sera, indicating that the immune responses are HIV Env specific (Fig. 2). To determine the antibody response to the V3 loop peptide, a strain-specific neutralizing epitope, antibody levels were analyzed using ELISA plates coated with the 89.6 V3 loop peptide. It is interesting to note that levels of V3-loop binding antibodies were significantly higher in mice immunized with dV1V2 mutant VLPs than with wild type although both groups were similar in levels of antibody binding to the whole HIV Env antigen (Fig. 2B).
Fig. 2.
IgG antibody responses against HIV Env after immunization with SHIV VLPs. A) HIV Env-specific total IgG antibody. Sera were collected before immunization and 2 weeks after every immunization. Results are expressed as the arithmetic mean ± SD of 100-fold diluted serum samples from 6 mice per group. Error bars indicate standard deviation (SD). The ANOVA test from the PC-SAS program showed significant differences between 3G-dV2−1G and WT (**: P < 0.001) and between WT and 3G (*: P <0.05). B) V3 loop peptide specific total IgG antibody. After the 2nd boost immunization, total IgG antibody binding to the V3 loop peptide (100 fold-diluted sera) was determined. Gag: HIV Env-negative SIV Gag VLPs, WT: SHIV VLPs with wild type HIV Env 89.6, dV1V2: SHIV VLPs with dV1V2 mutant HIV Env, 3G: SHIV VLPs with 3G mutant HIV Env, 3G-dV2−1G: SHIV VLPs with 3G-dV2−1G mutant HIV Env. (*: P < 0.05, **: P < 0.001).
When antibody responses against VLPs were determined by ELISA using wild type or mutant SHIV VLPs as coating antigens, no differences were observed in levels of binding antibody responses to VLP antigens (data not shown). Regarding the induction of serum IgG subtypes, IgG1, IgG2a, IgG2b, IgG3, and IgA antibodies binding to the HIV Env antigen were observed in all groups of mice immunized with wild type or mutant SHIV VLPs (data not shown), indicating that both T helper type 1 (Th1)- and Th2-like cellular immune responses are elicited. These results indicate that SHIV VLPs with modified Env protein are as immunogenic as wild type SHIV VLPs in inducing binding antibody to the HIV Env antigens.
3.3 Neutralization activities.
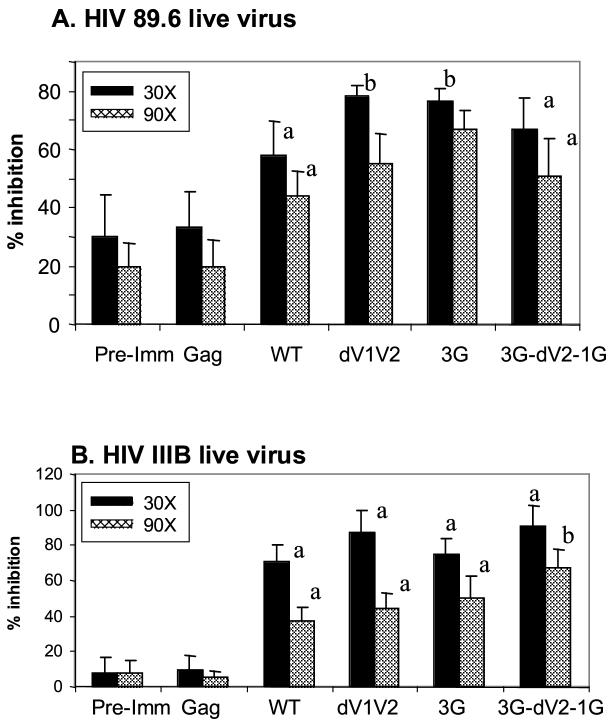
We determined neutralizing activities against the homologous strain, HIV 89.6 virus. Using luciferase reporter viruses pseudotyped with an HIV Env or live virus, normal mouse sera exhibit 20 to 50% background neutralization activities compared to immune sera [19, 27]. Similarly, we observed relatively high background neutralizing activity in pre-immune sera (Fig. 3). The immune sera from mice immunized with wild type SHIV VLPs showed significant increases in neutralization activity as compared with those observed with pre-immune sera (P < 0.05), whereas the immune sera of mice immunized with Env-negative SIV Gag VLPs showed similar levels of background neutralizing activity as pre-immune sera (Fig. 3). The 3G mutant SHIV VLPs induced the highest level of neutralization activities resulting in 80% reduction in infectivity, which is a significant increase compared to VLPs containing wild type Env (P < 0.05) (Fig. 3A). SHIV VLPs with a dV1V2 mutation also showed significantly higher neutralization activities than the wild type SHIV VLPs (P < 0.05).
Fig. 3.
Neutralization activities in sera of mice immunized with VLPs. To compare the neutralization activities in immune sera from groups, individual serum samples (six mice per group) after the 2nd boost were diluted to 30 (30X) and 90 times (90X). The neutralization activity was expressed as the percentage (%) of infectious spots of the immune serum samples as compared to those of control wells without serum samples and the activity of individual samples was determined as average from three independent experiments. Error bars indicate the standard deviation (SD). Statistically significant differences are indicated; between pre-immune sera and WT or mutants as “a” (P <0.05) and between WT and mutants as “b” (P <0.05). A) Neutralization activity against HIV 89.6. B) Neutralization activity against HIV IIIB. Immunization groups are the same as described in Fig. 2.
Next, we determined neutralization activities using YU2 pseudotyped virions, a primary isolate using CCR5 as a coreceptor [28]. The 3G-dV2−1G mutant VLP-immune sera showed the highest neutralization activity, with a reduction up to 60 % of YU2 infectivity (data not shown). In case of the laboratory-adapted, T cell-tropic IIIB strain virus using CXCR4 as a coreceptor [29] (Fig. 3B), immune sera from mutants dV1V2, 3G-dV2−1G, showed neutralizing activity up to 90% reduction against the IIIB strain. Overall, these results indicate that immunization with SHIV VLPs can induce neutralizing activities against homologous as well as heterologous strains and that mutated HIV Env containing variable loop deletions or deglycosylations presented in VLPs can enhance these neutralizing activities.
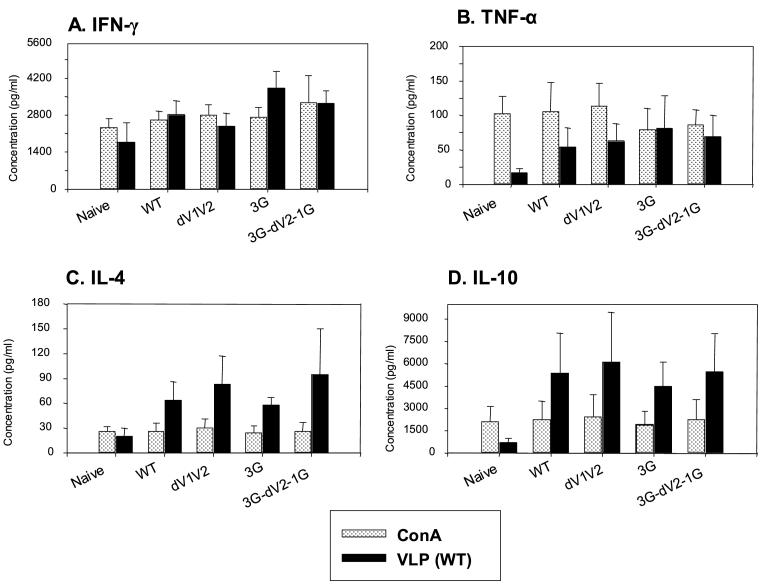
3.5 Cytokine responses induced by immunization with SHIV VLPs
An ideal vaccine against HIV-1 infection should elicit both humoral and cellular immune responses, which would provide protective immunity. To evaluate cellular immune responses, we determined the levels of a series of cytokines (IFN-γ, TNF-α, IL-4, and IL-10) produced by splenocytes from immunized mice. We compared cytokine responses stimulated using SHIV VLPs, a multi-epitope antigen and Con A, a strong T cell stimulator as a positive control. We observed that Con A stimulated splenocytes to secrete cytokines equally well from immunized and naïve mice, whereas stimulation with wild type SHIV VLPs resulted in differential effects on splenocytes in immunized and naïve mice (Fig. 4). IFN-γ was observed at moderately higher levels in mice immunized with 3G-dV2−1G VLPs than wild type or other mutant VLPs (Fig. 4A). Cytokines TNF-α, IL-4, and IL-10 were produced at 4 to 10-fold higher levels in SHIV VLP immunized mice than in naïve mice (Fig. 4B, C, D). Interestingly, SHIV VLP stimulated splenocytes to secrete IL-4 and IL-10 at significantly higher levels than Con A while other cytokines (IFN-γ, TNF-α) were produced at similar levels by both stimulators (Fig. 4). These results indicate that SHIV VLPs are a strong stimulator for inducing cytokine production.
Fig. 4.
Secretion of cytokines in responses to VLP stimulation. Bars indicate standard deviations from six mice per group. A) IFN-γ, B) TNF-α, C) IL-4, D) IL-10. Naïve: unimmunized mice, WT: immunized mice with wild type SHIV VLPs, dV1V2: immunized mice with dV1V2 SHIV VLPs, 3G: immunized with 3G SHIV VLPs, 3G-dV2−1G: immunized mice with 3G-dV2−1G SHIV VLPs.
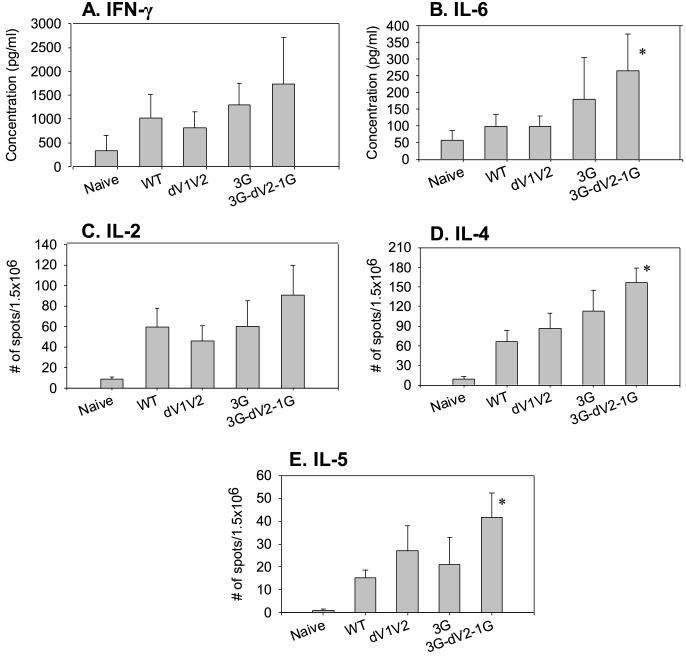
3.6 HIV Env peptide specific cytokine responses
To evaluate HIV Env peptide specific cytokine production, splenocytes from immunized or naïve mice were cultured in the presence of HIV Env 89.6 derived-peptide (Fig. 5). We observed approximately 4 to 5-fold increases in IFN-γ and IL-6 levels from the 3G-dV2−1G groups compared to the naïve group (Fig. 5A and 5B). All VLP-immunized groups showed over a 10-fold increase in numbers of cytokine (IL-2, 4, 5) secreting cells compared to the naïve mice (Fig. 5C, 5D, 5E). Mutant 3G-dV2−1G group increased IL-4, IL-5, and IL-6 secreting cells by over 2-fold compared to the wild type group (Fig. 5B, 5D, 5E), which is statistically significant (P < 0.05). Thus, these results indicate that SHIV VLPs can induce both Th1 and Th2 types of cellular immune responses, and that mutations in HIV Env affect the levels of cellular immune responses.
Fig. 5.
HIV Env peptide specific cytokines produced by spleen cells. A) IFN-γ ELISA. B) IL-6 ELISA. The culture supernatants of splenocytes after stimulation with an HIV Env 89.6 peptide were used to determine cytokines IFN-γ and IL-6 using ELISA. C to E) Cytokines (IL-2, 4, 5) determined by ELISPOT. The spots for cytokine-producing splenocytes after stimulation with an HIV Env 89.6 peptide were counted and expressed based on 1.5 × 106 cells. Bars indicate standard deviations. Immunization groups are the same as described in Fig. 4. Statistically significant differences between WT and mutants are indicated as * (P <0.05).
3.7 SHIV VLPs interact with antigen presenting cells.
To determine the phenotypes of cells binding to VLPs, biotin conjugated wild type, mutant SHIV VLPs, or SIV Gag VLPs (Env-negative control) were incubated with spleen cells, extensively washed to remove unbound VLPs, and then cells were stained with specific cell marker antibodies for CD4 and CD8 T cells, and CD19+ B cells (Fig. 6). Neither CD4 nor CD8 T cells showed significant binding to VLPs. Interestingly, VLPs were found to bind CD19+ B cells in a differential manner. Wild type 89.6 SHIV VLPs bound to B cells at 2 to 3 fold higher levels than those of Env-negative SIV Gag VLPs (Fig. 6J and 6K). Mutant 3G-dV2−1G bound to CD19+ B cells at a slightly higher level than that of wild type (Fig. 6K and 6L). Mutants dV1V2 and 3G VLPs showed similar levels of binding to CD19+ B cells as wild type VLPs (data not shown).
Fig. 6.
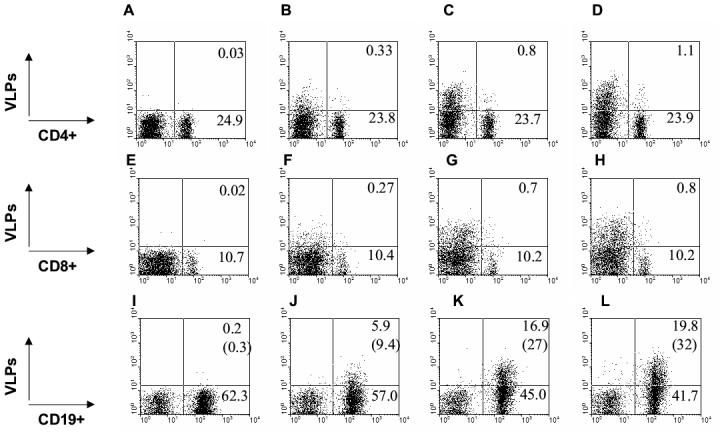
VLPs bind to splenic B cells. A to D) CD4+ cells binding VLPs. E to H) CD8+ cells binding VLPs. I to L) CD19+ cells binding VLPs. A, E, I) Unlabeled SHIV 89.6 VLPs. B, F, J) Biotinylated Gag VLPs without HIV Env. C, G, K) Biotinlyated SHIV 89.6 VLPs. D, H, L) Biotinylated 3G-dV2−1G SHIV VLPs. Numbers in each FACS profile indicate percentages in total gated populations and numbers in parentheses of I, J, K, L indicate percentages of gated CD19+ B cells. The FACS profiles shown in this figure are representative from four independent experiments.
To study VLP binding to dendritic cells (DCs), splenocytes with DC-enriched populations were obtained from mice injected with Flt3 ligand encoding DNA as described [25]. SHIV VLPs containing HIV Env showed higher levels of binding to CD11c+ populations compared to Env-negative Gag VLPs (Fig. 7B, C, and D). Mutant 3G-dV2−1G VLPs was moderately higher in binding to CD11c+ populations than wild type (Fig. 7D) and other SHIV VLPs (data not shown). When gated with MHCII+ populations, most of the VLP bound populations were MHCII+ phenotype (Fig. 7F, G, and H). Thus, the results indicate that VLPs interact with DCs and B cells, both of which are antigen-presenting cells.
Fig. 7.
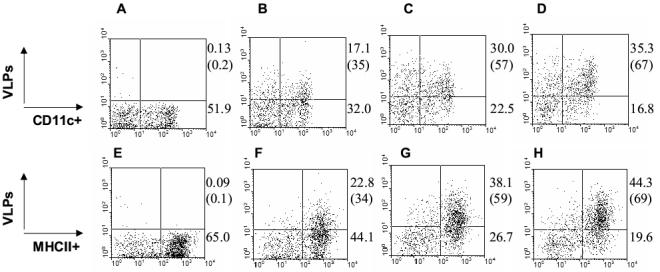
VLPs bind to dendritic (DCs) and MHCII+ cells. A to D) CD11c+ cells binding VLPs. E to H) MHCII+ cells binding VLPs. A, E) Unlabeled SHIV 89.6 VLPs, B, F) Biotinylated Gag VLPs without HIV Env. C, G) Biotinlyated SHIV 89.6 VLPs. D, H) Biotinylated 3G-dV2−1G SHIV VLPs. Numbers in each FACS profile indicate percentages in total gated populations and numbers in parentheses indicate percentage of gated CD11c+ and MHCII+ cells. The FACS profiles shown in this figure are representative from four independent experiments.
4. Discussion
HIV-1 Env mutants lacking the V1 and V2 loops or the V2 loop were reported to exhibit increased sensitivity to neutralization by antibodies directed against the V3 loop [30]. Serum from a monkey immunized with a V2 loop-deleted oligomeric HIV Env exhibited a slightly higher neutralizing antibody titer against the parent virus and moderately broader, albeit weak, neutralizing activity against a limited number of heterologous HIV-1 isolates [31]. In addition, we previously showed that higher levels of broadly neutralizing monoclonal antibodies (b12, 447−52D, 2G12, 2F5, 17b) bound to the V1-V2 loop-deleted HIV Env expressed on cell surfaces [17]. Consistent with these previous studies, the current study shows that the dV1V2 HIV Env on VLPs induced higher levels of antibodies binding to the V3 loop peptide antigen, and moderately enhanced neutralizing activity against the homologous strain 89.6 and the heterologous strain IIIB compared to VLPs containing unmodified 89.6 Env. Our results also suggest that deletion of the V1-V2 loops may play a role in exposing the V3 loop resulting in enhancement of antibodies against the V3 loop peptide, which may contribute to enhancing neutralizing activities. However, neutralizing activity against the YU2 primary isolate that is widely considered to be neutralization-resistant, was not improved by the V1-V2 loop deletion. This may be due to the fact that primary isolates have more cryptic neutralizing epitopes including the V3 loop than T cell tropic strains using CXCR4 as a coreceptor [32].
N-linked glycosylations on HIV Env are known to be engaged in shielding some important neutralizing epitopes. Elimination of the highly conserved glycans was demonstrated to render the mutants more susceptible to neutralization by antibodies against the CD4 binding site epitopes [33, 34]. Macaques infected with V3 loop glycan-deficient viruses elicited broadly neutralizing antibodies [35]. We found that VLPs containing a combination mutation 3G-dV2−1G exhibited moderately but significantly enhanced neutralizing activity against heterologous strains (YU2, IIIB). This may be due to high levels of binding antibodies coating the virion, a mechanism suggested previously [36]. These results suggest that further alterations in the Env structure may increase the breadth of neutralizing activity.
Soluble recombinant viral proteins frequently exhibit poor immunogenicity and inability to stimulate cellular immune responses. Thus, the co-administration of adjuvants is needed to initiate effective immune responses. Unfortunately, many experimental adjuvants (cholera toxin, monophosphoryl lipid A, CpG oligonucleotides) have potential side effects such as toxicity or inflammation, and thus are not currently licensed for use in human vaccines. This and other studies [3, 37-40] demonstrate that VLPs containing HIV Env can induce humoral and cellular immune responses without use of adjuvants. We also found that VLPs stimulate lymphocytes to secrete various cytokines (IFN-γ, TNF-α, IL-4, IL-10) as good as Con A, a well-known T lymphocyte stimulator. This is likely to be related to the particulate structure of VLPs. Interestingly, a combination mutant 3G-dV2−1G VLPs induced higher levels of certain cytokines (IL-4, IL-5, IL-6) in responses to HIV Env peptide stimulation than the wild type Env VLPs.
VLP-based vaccines are currently under investigation for several human viruses, such as hepatitis virus, papillomavirus, rotavirus, parvovirus, and influenza virus [5-8, 12, 13]. Understanding the possible underlying mechanisms of immunogenicity of VLP antigens will be informative for designing more effective vaccines. We investigated the phenotypes of immune cells interacting with VLPs and demonstrated, for the first time, that enveloped SHIV VLPs interact with CD19+ B cells as well as CD11c+ DC populations and that MHCII+ antigen presenting cells are more likely to bind SHIV VLPs. A recent study also showed an interaction of SHIV VLPs with in vitro derived human DCs although the phenotypes of cells were not characterized [41]. In addition, VLPs containing 3G-dV2−1G bound to CD11c+ DC populations at moderately higher levels than wild type or other mutant VLPs suggesting that Env mutations may affect interactions with antigen presenting cells. The higher binding level of this mutant to DC populations, the most potent antigen presenting cells, may have contributed to its enhanced immunogenicity.
In summary, we observed that design of VLPs containing modified HIV Env could be an effective strategy to develop vaccines inducing neutralizing activity as well as cellular immune responses against HIV-1, although further improvement is necessary to increase the breadth of neutralizing activity. Also, identifying immune cells interacting with HIV VLP antigens provides insight for better understanding VLP-induced immune responses and designing more effective HIV immunogens.
Acknowledgments
This work was supported in part by NIH/NIAID grants AI57015 (S.K.) and AI28147 (R.W.C.). Human HIV patient antibodies and purified HIV Env were obtained through the NIH AIDS Research and Reference Reagent Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Redfield RR, Wright DC, James WD, Jones TS, Brown C, Burke DS. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316(11):673–6. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- 2.Senior K. Adeno-associated virus vectors under scrutiny. Lancet. 2002;359(9313):1216. doi: 10.1016/S0140-6736(02)08258-2. [DOI] [PubMed] [Google Scholar]
- 3.Montefiori DC, Safrit JT, Lydy SL, Barry AP, Bilska M, Vo HT, et al. Induction of neutralizing antibodies and gag-specific cellular immune responses to an R5 primary isolate of human immunodeficiency virus type 1 in rhesus macaques. JVirol. 2001;75(13):5879–90. doi: 10.1128/JVI.75.13.5879-5890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellenberger D, Wyatt L, Li B, Buge S, Lanier N, Rodriguez IV, et al. Comparative immunogenicity in rhesus monkeys of multi-protein HIV-1 (CRF02_AG) DNA/MVA vaccines expressing mature and immature VLPs. Virology. 2005 doi: 10.1016/j.virol.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Jeong SH, Qiao M, Nascimbeni M, Hu Z, Rehermann B, Murthy K, et al. Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates. J Virol. 2004;78(13):6995–7003. doi: 10.1128/JVI.78.13.6995-7003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata K, Lechmann M, Qiao M, Gunji T, Alter HJ, Liang TJ. Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection. Proc Natl Acad Sci U S A. 2003;100(11):6753–8. doi: 10.1073/pnas.1131929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93(4):284–92. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 8.O'Neal CM, Clements JD, Estes MK, Conner ME. Rotavirus 2/6 viruslike particles administered intranasally with cholera toxin, Escherichia coli heat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J Virol. 1998;72(4):3390–3. doi: 10.1128/jvi.72.4.3390-3393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108(3):241–7. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 10.Lobue AD, Lindesmith L, Yount B, Harrington PR, Thompson JM, Johnston RE, et al. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine. 2006;24(24):5220–34. doi: 10.1016/j.vaccine.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 11.Antonis AF, Bruschke CJ, Rueda P, Maranga L, Casal JI, Vela C, et al. A novel recombinant virus-like particle vaccine for prevention of porcine parvovirus-induced reproductive failure. Vaccine. 2006;24(26):5481–90. doi: 10.1016/j.vaccine.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 12.Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23(50):5751–9. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 13.Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18(1):244–51. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- 14.Bures R, Gaitan A, Zhu T, Graziosi C, McGrath KM, Tartaglia J, et al. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2000;16(18):2019–35. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- 15.Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265(18):10373–82. [PubMed] [Google Scholar]
- 16.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393(6686):648–59. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang SM, Quan FS, Huang C, Guo L, Ye L, Yang C, et al. Modified HIV envelope proteins with enhanced binding to neutralizing monoclonal antibodies. Virology. 2005;331(1):20–32. doi: 10.1016/j.virol.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Jones DH, McBride BW, Roff MA, Maloney V, Farrar GH. Purification and characterization of simian immunodeficiency virus (SIVmac) envelope glycoprotein gp130 from virus-infected cells. Vaccine. 1994;12(3):250–58. doi: 10.1016/0264-410x(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 19.Kang SM, Guo L, Yao Q, Skountzou I, Compans RW. Intranasal immunization with inactivated influenza virus enhances immune responses to coadministered simian-human immunodeficiency virus-like particle antigens. J Virol. 2004;78(18):9624–32. doi: 10.1128/JVI.78.18.9624-9632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Q, Kuhlmann FM, Eller R, Compans RW, Chen C. Production and characterization of simian--human immunodeficiency virus- like particles. AIDS ResHumRetroviruses. 2000;16(3):227–36. doi: 10.1089/088922200309322. [DOI] [PubMed] [Google Scholar]
- 21.Guo L, Lu X, Kang SM, Chen C, Compans RW, Yao Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Virology. 2003;313(2):502–13. doi: 10.1016/s0042-6822(03)00372-6. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Cohen J, Hosmalin A, Cease KB, Houghten R, Cornette JL, et al. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988;85(9):3105–9. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan FS, Matsumoto T, Lee JB, Timothy O, Kim TS, Joo KH, et al. Immunization with Trichinella spiralis Korean isolate larval excretory-secretory antigen induces protection and lymphocyte subset changes in rats. Immunol Invest. 2004;33(1):15–26. doi: 10.1081/imm-120027681. [DOI] [PubMed] [Google Scholar]
- 24.Chackerian B, Haigwood NL, Overbaugh J. Characterization of a CD4-expressing macaque cell line that can detect virus after a single replication cycle and can be infected by diverse simian immunodeficiency virus isolates. Virology. 1995;213(2):386–94. doi: 10.1006/viro.1995.0011. [DOI] [PubMed] [Google Scholar]
- 25.Sailaja G, Husain S, Nayak BP, Jabbar AM. Long-term maintenance of gp120-specific immune responses by genetic vaccination with the HIV-1 envelope genes linked to the gene encoding Flt-3 ligand. J Immunol. 2003;170(5):2496–507. doi: 10.4049/jimmunol.170.5.2496. [DOI] [PubMed] [Google Scholar]
- 26.Yamshchikov GV, Ritter GD, Vey M, Compans RW. Assembly of SIV virus-like particles containing envelope proteins using a baculovirus expression system. Virology. 1995;214(1):50–58. doi: 10.1006/viro.1995.9955. [DOI] [PubMed] [Google Scholar]
- 27.Dong M, Zhang PF, Grieder F, Lee J, Krishnamurthy G, VanCott T, et al. Induction of primary virus-cross-reactive human immunodeficiency virus type 1-neutralizing antibodies in small animals by using an alphavirus-derived in vivo expression system. J Virol. 2003;77(5):3119–30. doi: 10.1128/JVI.77.5.3119-3130.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li A, Katinger H, Posner MR, Cavacini L, Zolla-Pazner S, Gorny MK, et al. Synergistic neutralization of simian-human immunodeficiency virus SHIV-vpu+ by triple and quadruple combinations of human monoclonal antibodies and high-titer anti-human immunodeficiency virus type 1 immunoglobulins. J Virol. 1998;72(4):3235–40. doi: 10.1128/jvi.72.4.3235-3240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolei A, Biolchini A, Serra C, Curreli S, Gomes E, Dianzani F. Increased replication of T-cell-tropic HIV strains and CXC-chemokine receptor-4 induction in T cells treated with macrophage inflammatory protein (MIP)-1alpha, MIP-1beta and RANTES beta-chemokines. Aids. 1998;12(2):183–90. doi: 10.1097/00002030-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Cao J, Sullivan N, Desjardin E, Parolin C, Robinson J, Wyatt R, et al. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. JVirol. 1997;71(12):9808–12. doi: 10.1128/jvi.71.12.9808-9812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett SW, Lu S, Srivastava I, Cherpelis S, Gettie A, Blanchard J, et al. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. JVirol. 2001;75(12):5526–40. doi: 10.1128/JVI.75.12.5526-5540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lusso P, Earl PL, Sironi F, Santoro F, Ripamonti C, Scarlatti G, et al. Cryptic nature of a conserved, CD4-inducible V3 loop neutralization epitope in the native envelope glycoprotein oligomer of CCR5-restricted, but not CXCR4-using, primary human immunodeficiency virus type 1 strains. J Virol. 2005;79(11):6957–68. doi: 10.1128/JVI.79.11.6957-6968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malenbaum SE, Yang D, Cavacini L, Posner M, Robinson J, Cheng-Mayer C. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. JVirol. 2000;74(23):11008–16. doi: 10.1128/jvi.74.23.11008-11016.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaffrey RA, Saunders C, Hensel M, Stamatatos L. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J Virol. 2004;78(7):3279–95. doi: 10.1128/JVI.78.7.3279-3295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malenbaum SE, Yang D, Cheng-Mayer C. Evidence for similar recognition of the conserved neutralization epitopes of human immunodeficiency virus type 1 envelope gp120 in humans and macaques. JVirol. 2001;75(19):9287–96. doi: 10.1128/JVI.75.19.9287-9296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burton DR, Saphire EO, Parren PW. A model for neutralization of viruses based on antibody coating of the virion surface. Curr Top Microbiol Immunol. 2001;260:109–43. doi: 10.1007/978-3-662-05783-4_7. [DOI] [PubMed] [Google Scholar]
- 37.Deml L, Schirmbeck R, Reimann J, Wolf H, Wagner R. Recombinant human immunodeficiency Pr55gag virus-like particles presenting chimeric envelope glycoproteins induce cytotoxic T-cells and neutralizing antibodies. Virology. 1997;235(1):26–39. doi: 10.1006/viro.1997.8668. [DOI] [PubMed] [Google Scholar]
- 38.Notka F, Stahl-Hennig C, Dittmer U, Wolf H, Wagner R. Accelerated clearance of SHIV in rhesus monkeys by virus-like particle vaccines is dependent on induction of neutralizing antibodies. Vaccine. 1999;18(3−4):291–301. doi: 10.1016/s0264-410x(99)00200-5. [DOI] [PubMed] [Google Scholar]
- 39.Buonaguro L, Racioppi L, Tornesello ML, Arra C, Visciano ML, Biryahwaho B, et al. Induction of neutralizing antibodies and cytotoxic T lymphocytes in Balb/c mice immunized with virus-like particles presenting a gp120 molecule from a HIV-1 isolate of clade A. Antiviral Res. 2002;54(3):189–201. doi: 10.1016/s0166-3542(02)00004-9. [DOI] [PubMed] [Google Scholar]
- 40.Buonaguro L, Visciano ML, Tornesello ML, Tagliamonte M, Biryahwaho B, Buonaguro FM. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 clade A virus-like particles administered by different routes of inoculation. J Virol. 2005;79(11):7059–67. doi: 10.1128/JVI.79.11.7059-7067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R, Li M, Chen C, Yao Q. SHIV virus-like particles bind and activate human dendritic cells. Vaccine. 2004;23(2):139–47. doi: 10.1016/j.vaccine.2004.05.036. [DOI] [PubMed] [Google Scholar]