The Keystone Symposium on Apoptotic and Non-Apoptotic Cell Death Pathways took place between 15 and 20 April 2007, in Monterey, CA, USA, and was organized by J. Yuan, D. Bredesen and Z. Zakeri.
Introduction
In an adult human, millions of cells die each second as a result of normal development as well as loss associated with pathology. For years, it has been widely assumed that this cell death occurs primarily through one mechanism—apoptosis—at least in physiological circumstances (Lockshin & Zakeri, 2001; Horvitz, 2003). The 2007 Keystone Symposium on Apoptosis and Non-Apoptotic Cell Death Pathways marked a turning point in the field by acknowledging the multiplicity of cell death mechanisms that exist. In most systems of apoptosis induction, the inhibition of caspases—cysteine proteases that are crucial to apoptosis—unmasks other mechanisms of cell death, which often shifts the apoptotic default pathway to non-apoptotic cell death modalities. Furthermore, such non-apoptotic cell death mechanisms occur in several in vitro and in vivo models of normal and pathological cell death (Yuan, 2003; Levine & Klionsky, 2004; Festjens et al, 2006; Gozuacik & Kimchi, 2007).
The length of time that it has taken for these non-apoptotic cell deaths to be fully accepted reflects the importance and impact of the initial discovery of apoptosis and its caspase-mediated mechanism. It might also reflect a certain reluctance at recognizing—even down one's own microscope—the existence of pathways that do not fit the established dogma. Meetings such as this symposium do much for the realization of the widespread occurrence of non-apoptotic cell death, and this should have therapeutic consequences.
A multiplicity of cell death pathways
The terms ‘programmed cell death' and ‘apoptosis' have been virtually synonymous for more than two decades. However, programmed cell death encompasses the cellular demise that results from an ordered, controlled process irrespective of the mechanism, whereas apoptosis describes a particular morphology of cell death, which is defined by nuclear and chromatin condensation (pyknosis) followed by nuclear fragmentation (karyorrhexis), and reflects one of several mechanisms of cell death (Fig 1; Kroemer et al, 2005; Bredesen, 2007). One of the most efficient ways to block apoptosis in vitro is the removal of the two principal pro-apoptotic multidomain proteins of the B-cell lymphoma 2 (Bcl2) family, Bax and Bak. Although more than 95% of Bax−/−Bak−/− mice die during birth or before weaning, C. Thompson (Philadelphia, PA, USA) pointed out that approximately 2–3% of the animals survive into adulthood without any significant developmental defect. Bax−/−Bak−/− cells are resistant to the induction of apoptosis through the intrinsic (mitochondrial) pathway, which suggests that apoptosis—or at least a large part of the apoptotic pathway—is not absolutely required for development and homeostasis. Therefore, if cell death is an absolute requirement for development, alternative routes to cell death must exist in mammals. In this context, it should be remembered that a primordial function of caspases might be cellular defence, at least in Caenorhabditis elegans, because caspase-deficient worms develop normally but are highly susceptible to infection. In mammals, apoptosis is also an important line of defence against infection, including viral infection, and interestingly viruses encode many anti-apoptotic proteins such as Bcl2-like molecules.
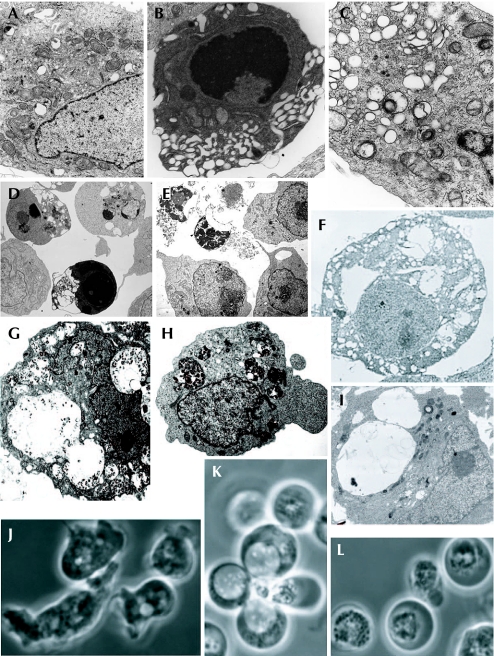
Figure 1.
Examples of names and types of cell death. (A–I) Electron microscopy; (J–L) phase contrast. (A) Normal HeLa cells; (B) treated with staurosporin to induce apoptosis; or (C) treated with thapsigargin to induce autophagy (Galluzzi et al, 2007). (D) Apoptotic, and (E) necroptotic Jurkat cells (Degterev et al, 2005). (F) Paraptotic 293T cells (Sperandio et al, 2000; copyright (2000) National Academy of Sciences, USA). (G) Necrotic and (H) autophagic iBMK cells (Degenhardt et al, 2006; Mathew et al, 2007). (I) Ras-expressing U251 glioblastoma cells showing macropinosomes (J.H. Overmeyer, A. Kaul, E.E. Johnson & W.A. Maltese, unpublished data). (J) Dictyostelium cells, vegetative, (K) undergoing vacuolar autophagic cell death, and (L) undergoing necrotic cell death (Laporte et al, 2007).
Ironically, the term ‘programmed cell death' was coined during the observation of intersegmental muscle cells of Manduca sexta, a type of cell that manifests massive autophagic vacuolization—the digestion of part of a cell, which can either rescue the cell or lead to its death—before the cells show signs of apoptosis (Beaulaton & Lockshin, 1977). This raises questions about the relationship between autophagy and apoptosis, as pointed out by R. Lockshin (Jamaica, NY, USA). In addition, when the salivary gland of a developing fruit fly (Drosophila melanogaster) undergoes involution, its cells undergo massive autophagic vacuolization. E. Baehrecke (College Park, MD, USA) reported that transgenic expression of the caspase inhibitor p35 in the salivary gland prevents nuclear pyknosis—a sign of apoptosis—but does not affect vacuolization—a sign of autophagy—placing autophagy either upstream from or parallel to apoptosis. Transgenic expression of inhibitors of autophagy enhances the growth of salivary gland cells, but fails to prevent caspase activation and the degradation of the nuclear envelope and DNA. Similarly, mutations in essential autophagy genes fail to prevent changes that occur during cell death. These results indicate that autophagy is probably dispensable for cell death, although, in this system, it can contribute to the rapid degradation of the cells that are primed to die. B. Levine (Dallas, TX, USA) presented data on mouse embryoid body cavitation that suggest that in dying cells in which autophagy occurs before apoptosis, autophagy is a mechanism through which the cells generate sufficient ATP to emit ‘come and get me'—that is, lysophosphatidylcholine secretion—and ‘eat me'—that is, phosphatidylserine exposure—signals to neighbouring cells.
E. White (Piscataway, NJ, USA) showed that immortalized epithelial cells die by apoptosis in response to metabolic stress—for example, glucose depletion and hypoxia—in a Bax/Bak-dependent manner. She presented convincing evidence that, in this case, autophagy is mainly a survival pathway: specifically, the genetic ablation of autophagy by the allelic loss of beclin 1 (an essential mediator of autophagy that also interacts with members of the Bcl2 family), the knockdown of beclin 1 or autophagy gene 5 (atg5, another mediator of autophagy) by RNA interference, or a deficiency in atg5 impaired survival of Bax−/−Bak−/− cells or Bcl2-expressing tumour cells subjected to metabolic stress. Under these conditions, death by apoptosis and survival by autophagy are not possible, and tumour cells undergo rapid cell death by necrosis (Degenhardt et al, 2006). Overall, the prevailing consensus that emerged at the conference was that cells rarely die through autophagy and that in those cases in which cell death is accompanied by autophagy, this often constitutes a defence mechanism against stress. This conclusion was supported by the electron microscopy data of E.-L. Eskelinen (Helsinki, Finland), who frequently detected autophagosomes—defined as vacuoles containing cytoplasmic material and surrounded by a double membrane—in the absence of cell death.
J. Overmeyer (Toledo, OH, USA) presented a model of vacuolization-associated cell death that is not related to autophagy. She showed that transfection with activated Ras (RasG12V) can induce U251 glioblastoma cells to die and that they accumulate vacuoles that exclude the autophagy marker LC3 fused to green fluorescent protein. These vacuoles are rapidly labelled with extracellular fluid-phase tracers, such as Lucifer yellow and dextran-AlexaFluor 488, which suggests that they are macropinosomes. Later in the process, the cells detach from the substrate and then display evidence of caspase activation. These results illustrate that prominent vacuolization is not always related to autophagy.
Several speakers, in particular J. Yuan (Boston, MA, USA), provided examples of ‘necroptosis'. This process can occur when death receptors such as Fas/CD95 or the tumour necrosis factor receptor (TNFR) are activated by their ligands—which usually induces apoptosis—while caspase activation is inhibited. This causes a shift from apoptosis to non-apoptotic cell death with features of autophagy and necrosis. Depending on the specific experimental conditions, this can lead to autophagic catabolism. M. Lenardo (Bethesda, MD, USA) reported that inhibition of caspase 8 was sufficient to induce atg7-dependent autophagic cell death, at least in L929 fibrosarcoma cells. He also indicated that this autophagy led to the selective degradation of catalase, apparently in lysosomes, and that this loss might be sufficient to explain the subsequent production of reactive oxygen species and the cell death that is associated with autophagy. D. Bredesen (Novato, CA, USA) suggested that a combination of caspase and calpain inhibition would be particularly efficient in triggering this type of cell death, for example, to eliminate apoptosis-resistant tumour cells.
P. Golstein (Marseille, France) reported that cells from the slime mould Dictyostelium discoideum, a genetically tractable model with no potentially interfering apoptosis machinery, undergo necrosis when the atg1 autophagy gene is inactivated and the cells are subjected to a standard developmental stimulus. The cells then manifest a stereotypical pattern of alterations: within 1–3 min they show an increase in oxygen consumption, an oxidative burst within mitochondria and ATP depletion; within 20–30 min mitochondria cluster in the perinuclear area; within 90 min lysosomal lesions appear; and at around 150 min plasma membranes rupture. The uncouplers dinitrophenol and carbonyl cyanide m-chlorophenylhydrazone induce the same effects as the physiological differentiation factor DIF, suggesting that mitochondrial alterations have an important role in this type of physiologically triggered necrosis. In agreement with this idea, Y. Tsujimoto (Osaka, Japan), as well as several other groups, showed that mice lacking one particular mitochondrial protein, cyclophilin D, exhibit marked resistance to necrotic cell death, particularly when induced by ischaemia in the heart or brain.
M. Driscoll (Piscataway, NJ, USA) showed that mutations in the ion channels degenerins can induce necrosis in the neurons of C. elegans. This is due to the locking of the channels in an open state, presumably by increasing Na+ and Ca2+ fluxes. In addition, the deletion of glutamate transporters causes an increase in glutamate concentration, thus inducing excitotoxic neuronal cell death. The individual deletion of several proteases—three calpains and two cathepsins—or that of several proteins involved in endoplasmic reticulum Ca2+signalling—that is, calreticulin, the IP3 receptor or the ryanodine receptor—reduced the observed necrosis. Therefore, endoplasmic reticulum Ca2+ stores are crucial for this neurotoxicity, which apparently involves terminal lysosome disruption.
V. Dawson (Baltimore, MD, USA) discussed ‘Parthanatos', a caspase-independent cell death modality that is characterized by the condensation of the nucleus to a very small size. Parthanatos involves the activation of polyadenosine ribose polymerase (PARP), the generation of polyadenosine ribose (PAR) polymers in the nucleus, their translocation to mitochondria, PAR-mediated release of apoptosis-inducing factor (AIF) from mitochondria, the translocation of AIF to the nucleus and AIF-dependent, caspase-independent chromatin condensation. Dawson identified a new protein that she named Iduna, which can bind to and neutralize PAR in the cytosol. This suggests that the amounts of PAR, and therefore resulting cell death, are finely regulated.
J. Brugge (Boston, MA, USA) reported on yet another system of cellular demise—entosis—in which one cell invades another cell, even while it is still alive. She used a three-dimensional culture system of breast epithelial acini, which recapitulates some aspects of mammary gland development, including the formation of luminal space within the acini. Three mechanisms seem to contribute to the clearance of the matrix-deprived cells in the presumptive luminal space of the three-dimensional structures, and all of them can be observed in a simpler model in which cells are cultured in suspension in the absence of extracellular matrix proteins. Apoptosis—which, in this system, requires two BH3-only proteins, Bim and Bmf—represents the main mechanism of cell clearance. Autophagy, apparently as a result of metabolic impairment, is prominent and might maintain the viability of cells until they can intercalate into the outer cell layer and be rescued by matrix attachment. The third clearance mechanism—entosis—involves heterophagy, which is not inhibited by Bcl2 or Z-VAD-fmk (a caspase inhibitor), and the internalized cells appear virtually normal. Most eventually disappear, presumably through lysosomal degradation; however, in rare cases, the internalized cells divide within the engulfing cells or are released. Cells with similar cell-in-cell morphological features are commonly detected in fluid exudates, and in solid tumours and leukaemia. In addition, cell-in-cell structures were also described by Z. Zakeri (Flushing, NY, USA) in influenza-virus-infected mouse embryonic fibroblasts that lack cathepsin B, D or L.
Crosstalk between different cell death modalities
A. Kimchi (Rehovot, Israel) described a system-level analysis based on multiple combinatorial perturbations, which quantifies the contribution and crosstalk between different cell death modalities. She reported on effector/signalling proteins common to both apoptosis and autophagy that might function as coordinators or molecular switches between cell death modalities. These include members of the DAP kinase (DAPk) family of proteins and isoforms of the cell cycle inhibitor p19ARF. Kimchi showed that a pro-autophagic short isoform of p19ARF interacts with the mitochondrial p32 protein, dissipates mitochondrial membrane potential and causes caspase-independent cell death. DAPk knockout increases cell survival by reducing both caspase activation and autophagy in response to endoplasmic reticulum stress. P. Codogno (Chatenay Malabry, France) showed that sphingolipids can modulate both autophagy and apoptosis, and that ceramide is a strong inducer of both autophagy and apoptosis.
F. Cecconi (Rome, Italy) reported on the characterization of a new protein, ambra 1, which can interact with beclin 1 to stimulate autophagy, probably by favouring its interaction with the lipid kinase Vacuolar protein sorting-associated protein 34 (Vps34). Ambra 1 is mainly expressed in the nervous system during development, but is ubiquitous in adults. The knockout of ambra 1 inhibits autophagy induced by rapamycin or starvation, and causes excessive cell death as well as excessive proliferation in the neuronal system. It also leads to the accumulation of ubiquitinated proteins in neuroepithelia, failure of the neural tube to close, and exencephaly in the mid–hind-brain region and hyperplasia of basal ganglia.
G. Kroemer (Villejuif, France) suggested that some BH3-only proteins that are notorious for inducing apoptosis can trigger autophagy by competitively inhibiting the interaction between beclin 1 and the multidomain anti-apoptotic proteins of the Bcl2 family, in particular Bcl2 and Bcl-XL. This would lead to the release of beclin 1 from its inhibition by Bcl2/Bcl-XL, and allow it to act as an allosteric activator of the lipid kinase Vps34, which in turn is required for autophagy induction. The structural basis of the interaction between beclin 1 and Bcl-XL is the BH3 domain in beclin 1, an amphiphatic α-helix that binds to the hydrophobic groove (the BH3-receptor domain) of Bcl-XL.
Levine showed that starvation reduces the interaction in co-immunoprecipitation assays between Bcl2 and beclin 1. She showed that the c-Jun amino-terminal kinase (JNK) is stimulated by nutrient depletion, which leads to the phosphorylation of Bcl2 on three residues—threonine 69, serine 70 and serine 87—in its flexible loop. Accordingly, phosphomimetic mutations of the Bcl2 loop reduce the interactions of beclin 1 and Bcl2, and abolish the capacity of Bcl2 to inhibit autophagy, whereas non-phosphorylatable Bcl2 remains bound to beclin 1 under conditions of starvation. Constitutively active JNK blocks the interaction between Bcl2 and beclin 1, and a dominant-negative JNK inhibits the starvation-induced autophagy and the dissociation of Bcl2 and beclin 1. It is unclear how this mechanism relates to the regulation of apoptosis by BH3 domains.
White provided a mechanistic explanation for why beclin 1 is a haplo-insufficient tumour suppressor—that is, through a mismanagement of metabolic stress that promotes tumorigenesis. DNA-damage foci, supernumerary centrosomes, double minute chromosomes and aneuploidy are far more common in Bcl2-expressing, autophagy-defective beclin 1+/− cells than in beclin 1+/+ cells. These data suggest that the combined inhibition of apoptosis and autophagy facilitates chromosomal instability. Similar results are obtained with atg5-deficient cells, indicating that perturbations of the autophagic pathway can favour genomic instability.
Lenardo showed that in L929 cells treated with the caspase inhibitor Z-VAD-fmk, there is a translocation to lysosomes of the BH3-only protein hSpin, a protein that probably participates in the fusion between autophagosomes and lysosomes. hSpin depletion prevents the Z-VAD-fmk-induced autophagy and the degradation of catalase that might finally cause cell death in this model.
S. Kornbluth (Durham, NC, USA) showed that NADPH inhibits caspase 2 activation in Xenopus egg extracts. NADPH-stimulated Ca2+/calmodulin-dependent protein kinase II (CaMKII) phosphorylates caspase 2 at serine 135, and this phosphorylation is responsible for caspase 2 inhibition by NADPH. Importantly, glucose-6-phosphate not only induces caspase 2 phosphorylation but also inhibits its dephosphorylation by protein phosphatase 1. These results suggest how bioenergetic stress can induce apotosis. If autophagy is a mechanism that maintains high levels of redox equivalents, including NADPH, in stressed cells, then its inhibition would indirectly activate the caspase machinery; this hypothesis remains to be confirmed.
Together, the presentations at this meeting that addressed interdeath crosstalk revolved mostly around the Bcl2 family, probably because of its many intra- and extra-family connections, as also indicated by the non-lethal functions of the family members.
Lethal functions for vital proteins and vice versa
Several speakers suggested that proteins that act in the regulation or execution of cell death have other unrelated functions.
Tsujimoto reported that cyclophilin-D-deficient mice exhibit a deficit in learning and memory as well as motion. This might be related to an essential contribution of cyclophilin D, not only to necrotic cell death, as indicated above, but also to long-term potentiation, which is required for synaptic plasticity. Driscoll discovered that the C. elegans caspase CED-3 is required for the efficient regeneration of neurons that have been subjected to laser axotomy. Similarly, M. Miura (Tokyo, Japan) showed that the apical caspase DRONC—which activates the CED-3-related caspase DRICE during apoptosis induction—is required for compensatory proliferation in the imaginal discs of Drosophila. J.M. Hardwick (Baltimore, MD, USA) revealed the characterization of a conditional, neuron-specific knockout of Bcl-XL. Bcl-XL−/− neurons exhibit an elevated and unstable mitochondrial transmembrane potential (ΔΨm) as well as an increase in mitochondrial NADPH. The predominant localization of Bcl-XL in the normal mouse brain is on the inner mitochondrial membrane, where it might interact with the ATP synthase β-subunit. Hardwick formulated the possibility that Bcl-XL reduces the coupling between ATP synthesis and proton consumption under conditions of stress. Alternatively, she proposed that the normal function of Bcl-XL—its ‘day job'—might be to regulate cellular bioenergetics rather than apoptosis.
J. Lieberman (Boston, MA, USA) showed how granzyme A—a protease contained in the granules of cytotoxic T cells and natural killer cells—destroys the repair function of the DNA-repair complex SET and converts it into a DNA-destroying agent. This effect of granzyme A involves the direct proteolytic cleavage of a component of respiratory chain complex I, which is located in the mitochondrial matrix. This causes an increase in the production of reactive oxygen species, which triggers the relocation of the SET protein complex from the endoplasmic reticulum to the nucleus. Within the SET polyprotein complex, granzyme A cleaves APE1, SET and high mobility group box 2 (HMGB2), and therefore activates both the endonuclease NM23-H1 to generate DNA nicks and TREX1 to extend them. Granzyme A thus leads to both the nuclear translocation and the destructive alteration of the SET complex.
D.R. Green (Memphis, TN, USA) revealed a new and surprising function for glyceraldehyde phosphate dehydrogenase (GAPDH). On overexpression, GAPDH allows cells to survive a particular combination of conditions in which caspases are inhibited and cytochrome c is released from mitochondria—for example, after treatment with actinomycin D or staurosporine. GAPDH enhances glycolysis and stimulates autophagy through an increase in ATG12 expression. GAPDH hence induces a reduction in mitochondrial mass, perhaps owing to the selective autophagy of permeabilized mitochondria, which can be inhibited by ATG5 knockdown and which is required for its rescuing effect. Green suggested that a few mitochondria that remain ‘sealed' owing to the absence of the translocation of Bax could restore the bioenergetic function of the cell in these circumstances.
Targeting specific cell death pathways
The multiplicity of cell death pathways and relevant proteins provides additional opportunities to develop new strategies for therapeutic induction or inhibition of cell death. Accordingly, several speakers revealed new pharmacological approaches to induce or to inhibit cell death.
Yuan presented a screen for cytoprotective compounds that led to the identification of the necrostatins, a chemically heterogeneous collection of compounds that prevent necroptosis but not apoptosis. Necrostatin 1 reduces infarct areas in a mouse model of stroke, a manipulation that inhibits signs of autophagy but does not prevent caspase activation. Necrostatin 1 synergizes with Z-VAD-fmk for the reduction of infarct size in vivo, suggesting that the simultaneous inhibition of several cell death pathways should be the therapeutic aim for optimal neuroprotection.
S. Wang (Ann Arbor, MI, USA) presented data on a monovalent Smac mimetic (SM-122) that can bind to both the BIR2 and BIR3 domains of several inhibitor of apoptosis (IAP) proteins, including XIAP, cIAP1 and cIAP2. A bivalent compound (SM-164) binds with much higher affinity than SM-122 to XIAP. In both a cell-free system and intact cells, SM-164 is over 100 times more efficient as a caspase activator than SM-122. These agents can kill certain cancer cells (MDA-MB231, SKOV, MAMEL-3M, OVAR-4) with an IC50 (IC for inhibitory concentration) of 1–3 nM, but have no effect on approximately 80% of the cancer cells tested. A strategy for sensitizing cancer cells to SM-164 might involve the inhibition of Bcl2 or the Bcl2 family member Mcl1.
S.W. Fesik (Abott Park, IL, USA) reported on ABT263, a second-generation Bcl2 inhibitor that is available orally and has been introduced into phase I clinical trials. ABT263 binds to Bcl2, Bcl-XL, Bcl-w—but not Mcl1 or A1—and is efficient as a single agent against small cell lung cancer (SCLC) cell lines, and primary lymphoma and leukaemia cells. ABT263 is well tolerated in preclinical models but has haematological side effects (lymphopaenia and thrombocytopaenia). When ABT263 was tested on other cell lines, Mcl1 expression correlated with resistance, whereas expression of the Bcl2 family member Noxa correlated with sensitivity. Mcl1 knockdown sensitizes resistant cells to ABT263. Mcl1 can be inhibited by DNA damage (which upregulates Puma, another Bcl2 family member- and Noxa), cyclin-dependent kinase 1 (Cdk1) inhibition (which modifies Mcl1 levels), and bortezomib (which increases Noxa). This might explain why ABT263 can synergize with such treatments for the induction of tumour cell death.
M. Jäättelä (Copenhagen, Denmark) suggested that cancer cells have developed a particular strategy to suppress the lethal consequences of lysosomal membrane permeabilization (LMP), a non-apoptotic mechanism of cell death. Such cells might upregulate protease inhibitors, such as cystatin, serpinB3, serpinB4, hurpin and Spi2A, and stabilize lysosomes by upregulating the heat-shock proteins Hsp70-1 and Hsp70-2. In cancerous, but not normal cells, Hsp70 localizes to lysosomal membranes, presumably by direct binding of its amino terminus to an anionic lipid, lysobisphosphatidic acid. The local presence of Hsp70 stabilizes lysosomal membranes and avoids LMP. A new Hsp70 inhibitor, ADD70, inhibits the LMP-inhibitory effect of Hsp70 and is an efficient chemosensitizer. Jäättelä also reported on siramesine, a direct inducer of LMP that has potent anti-cancer effects as a monotherapy on xenografts of MCF-7 cells.
Thompson developed two approaches to modulate autophagy for the treatment of cancer. In one model, he showed that inhibition of autophagy by chloroquine can ameliorate the therapeutic outcome. Therefore, in c-Myc-induced lymphoma, reactivation of a latent p53 transgene has a therapeutic effect that is ameliorated by chloroquine. Thompson also showed that induction of autophagy might have an anti-cancer effect. HCT116 p53−/− cells—but not HCT116+/− controls—xenografted into immunodeficient mice are slow to grow after treatment with metformin or 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), which correlates with enhanced autophagy. Both metformin and AICAR are activators of AMP-activated protein kinase and are therefore inducers of autophagy.
These examples illustrate how the detailed knowledge of apoptotic and non-apoptotic cell death pathways can guide the development of new therapeutic strategies. They indicate the main directions of development of the field: towards new models and new fundamental findings on basic cell death mechanisms, and towards directly derived therapeutic advances.

Pierre Golstein

Guido Kroemer
Acknowledgments
We thank the organizers and all the speakers for an exciting meeting, and we apologize to the authors of those presentations that we could not discuss because of space limitations. We are supported by the European Community, Ligue Nationale Contre le Cancer, Association pour la Recherche sur le Cancer, Agence Nationale pour la Recherche, Institut National Contre le Cancer (INCa), Ministère pour la Recherche and Cancéropôles Ile-de-France, and Provence–Alpes–Côte d'Azur Region.
References
- Beaulaton J, Lockshin RA (1977) Ultrastructural study of the normal degeneration of the intersegmental muscles of Anthereae polyphemus and Manduca sexta (Insecta, Lepidoptera) with particular reference of cellular autophagy. J Morphol 154: 39–57 [DOI] [PubMed] [Google Scholar]
- Bredesen DE (2007) Keynote lecture: toward a mechanistic taxonomy for cell death programs. Stroke 38: 652–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K et al. (2006) Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10: 51–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of non-apoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1: 112–119 [DOI] [PubMed] [Google Scholar]
- Festjens N, Vanden Berghe T, Vandenabeele P (2006) Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta 1757: 1371–1387 [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G (2007) Cell death modalities: classification and pathophysiological implications. Cell Death Differ 14: 1237–1243 [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A (2007) Autophagy and cell death. Curr Top Dev Biol 78: 217–245 [DOI] [PubMed] [Google Scholar]
- Horvitz HR (2003) Nobel lecture. Worms, life and death. Biosci Rep 23: 239–303 [DOI] [PubMed] [Google Scholar]
- Kroemer G et al. (2005) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 12: 1463–1467 [DOI] [PubMed] [Google Scholar]
- Laporte C, Kosta A, Klein G, Aubry L, Lam D, Tresse E, Luciani MF, Golstein P (2007) A necrotic cell death model in a protist. Cell Death Differ 14: 266–274 [DOI] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ (2004) Development by self-digestion; molecular mechanisms and biological functions of autophagy. Dev Cell 6: 463–477 [DOI] [PubMed] [Google Scholar]
- Lockshin RA, Zakeri Z (2001) Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol 2: 545–550 [DOI] [PubMed] [Google Scholar]
- Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E (2007) Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 21: 1367–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio S, de Belle I, Bredesen DE (2000) An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA 97: 14376–14381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Lipinski M, Degterev A (2003) Diversity in the mechanisms of neuronal cell death. Neuron 40: 401–413 [DOI] [PubMed] [Google Scholar]