Abstract
The chaperonin GroEL binds nonnative proteins in its central channel through hydrophobic interactions and initiates productive folding in this space underneath bound cochaperone, GroES, in the presence of ATP. The questions of where along the folding pathway a protein is recognized by GroEL, and how much structure is present in a bound substrate have remained subjects of discussion, with some experiments suggesting that bound forms are fully unfolded and others suggesting that bound species are partially structured. Here we have studied a substrate protein, human dihydrofolate reductase (DHFR), observing in stopped-flow fluorescence experiments that it can rapidly bind to GroEL at various stages of folding. We have also analyzed the structure of the GroEL-bound protein using hydrogen–deuterium exchange and NMR spectroscopy. The pattern and magnitude of amide proton protection indicate that the central parallel β-sheet found in native DHFR is present in a moderately stable state in GroEL-bound DHFR. Considering that the β-strands are derived from distant parts of the primary structure, this suggests that a native-like global topology is also present. We conclude that significant native-like structure is present in protein-folding intermediates bound to GroEL.
Recent structural and functional studies of the Escherichia coli chaperonin GroEL and its small, single-ring cooperating component, GroES, indicate that GroEL provides a specialized environment, its central channel, favoring protein folding (1–3). The central channel is the site of two principal actions: binding of nonnative forms and facilitation of folding to the native state. Binding involves localization of nonnative polypeptide in the open channel (45 Å diameter) (4), where interactions form between hydrophobic side chains in the GroEL apical domains facing the channel and, presumably, hydrophobic, aggregation-prone surfaces of nonnative polypeptide (5–7). Facilitation of folding occurs in the central channel after binding of GroES to the same ring as polypeptide in the presence of ATP (3). GroES binding is associated with a major conformational change of the apical domains, permitting GroES to interact with the apical peptide binding sites and releasing polypeptide into an enlarged central channel (≈60 Å diameter × 60 Å height) (8–12), initiating folding in a sequestered space (2, 3). This folding–active complex has a half-life of ≈15 sec before it is dissociated by ATP hydrolysis in the ring opposite polypeptide and GroES (3, 13). During this time, a fraction of protein reaches native state or a form committed to it upon release, while another fraction is released in nonnative form and must be rebound for a further attempt at reaching the native state (3, 13–18).
While the folding–active state and end-products of the chaperonin reaction have been defined, the points on the folding pathway at which substrate is bound and the conformation of the substrate bound in the central channel have been a matter of debate. For example, a deuterium exchange study examining GroEL-bound cyclophilin concluded that the bound protein was fully unfolded (19), while an examination of scrambled 3-disulfide forms of α-lactalbumin bound to GroEL using deuterium exchange and mass spectrometry concluded that unstable secondary structure might be present (20). By contrast, a similar study of thermally destabilized β-lactamase bound to GroEL showed properties similar to the native state (21). An NMR study of small peptides transiently bound by GroEL observed that α-helical secondary structure could be acquired upon binding (22). To date, however, the structure of a substrate protein stably bound to GroEL has not been determined. Here we have employed stopped-flow fluorescence to observe the points during the refolding of dihydrofolate reductase (DHFR) diluted from denaturant at which GroEL binds to DHFR and arrests its folding, and hydrogen exchange and two-dimensional NMR spectroscopy to analyze the structure of GroEL-bound DHFR.
METHODS AND MATERIALS
Preparation of Proteins.
· 15N-labeled DHFR was purified as described (23). GroEL was expressed and purified as described (4). GroES was expressed in E. coli from a GroES-containing derivative of pET11a, kindly provided by J. Flanagan (Brookhaven National Laboratory), and purified as in ref. 13.
Fluorescence Spectroscopy.
Fluorescence data were collected on a Biologic (Grenoble, France) SFM3 stopped-flow fluorescence spectrometer exciting at 295 nm and detecting at all wavelengths over 320 nm with a cut-off filter. DHFR was incubated in 6.0 M urea for 3 hr at 15°C to ensure complete unfolding. Refolding was initiated by diluting 36 μl of this solution with 324 μl of buffer at 15°C; the dead time of mixing was 5 msec. For refolding in the presence of GroEL, the final concentrations of DHFR and GroEL were both 1.2 μM for the 400-sec trace and 2.7 μM for the 0.5-sec trace. The refolding buffer contained 20 mM potassium phosphate (pH 6.0), 100 mM KCl, 20 μM NADPH, 1 mM 2-mercaptoethanol, and 10-fold excess folate over protein concentration (to inhibit aggregation). For the double mixing experiments, the refolding buffer was identical except that the final folate concentration was 30 μM, the final DHFR concentration was 1.0 μM, and the final GroEL concentration was 1.2 μM. The data were collected as described above except that refolding was initiated by a 10-fold dilution of the unfolded protein before adding the GroEL at various times, producing a further 2-fold dilution with a mixing time of 30 msec. The final urea concentration was 0.6 M for the first stage of folding and 0.3 M after the GroEL was injected.
Hydrogen Exchange.
DHFR–GroEL binary complex was formed at 22°C by rapidly diluting 2 ml DHFR (10 mg/ml, unfolded in 6 M guanidine·HCl/50 mM Tris, pH 7.4/5 mM DTT) into 120 ml 25 mM potassium phosphate (pH 6.0), 20 mM KCl, and 1 mM DTT containing a 2-fold excess of GroEL (final concentrations, 8 μM DHFR, 16 μM GroEL tetradecamer ≈1.6 g). Under these conditions all of the input DHFR associates with GroEL and remains tightly bound in the absence of ATP. The binary complex was concentrated to 15 ml by ultrafiltration. For exchange, this solution was mixed with 120 ml 99.9% 2H2O (Isotech), 30 mM potassium phosphate, pH* 6.0 (uncorrected glass-electrode reading), 1 mM DTT at 15°C using a continuous flow mixing apparatus consisting of two Lee micromixers (Lee, Westbrook, CT) separated by a variable length delay line. Exchange was terminated in the second mixer by the addition of 10 ml 89% 2H2O Mg·ATP, GroES and methotrexate (MTX) (final concentration, 5 mM MgCl2, 2 mM ATP, 14 μM GroES, 70 μM MTX, pH* 6.0 at 15°C). All solutions were pumped by computer-controlled stepper motors and maintained at 15°C by submersion in a thermostated circulating water bath. This procedure was repeated for each of 10 identical samples with delay lines chosen to expose the GroEL-bound intermediate to 89% 2H2O for 0.3, 1.5, 4, 11, 27, 68, 182, 452, 1200, and 3000 sec. Manual mixing was employed for the four longest exposure times. After the exposure to 89% 2H2O was terminated, samples were allowed to refold for 20 min, then shifted to 4°C for all subsequent steps. GroEL and GroES were separated from native DHFR–MTX by anion exchange chromatography (Poros 50 HQ resin; PerSeptive Biosystems, Cambridge, MA). DHFR–MTX was collected from the flow-through fractions, while GroEL and GroES required a 0–1 M KCl gradient for elution. The purified DHFR–MTX was concentrated to 2 ml by ultrafiltration, and transferred to 99.9% 2H2O 50 mM potassium phosphate pH* 6.5, 25 mM KCl by repeated dilution and concentration. Upon concentration to a final volume of 400 μl, samples were centrifuged to remove debris, frozen, and stored at −80°C until NMR data acquisition. Final sample concentrations were 1–2 mM. To determine the amide exchange rate constants of native DHFR complexed with MTX, native fully protonated 15N-labeled DHFR–MTX was transferred into 2H2O by chromatography on a PD-10 column (Pharmacia) equilibrated with 99.9% 2H2O 50 mM potassium phosphate pH* 6.5, 25 mM KCl. The sample was concentrated to 400 μl, and NMR spectra were acquired at 25°C after 3, 6, 9, 12, 14, 17, 20, 244, and 436 h.
NMR Spectroscopy.
For all samples, two-dimensional 1H-15N heteronuclear single quantum coherence spectra were collected at 25°C on a Varian UNITYplus 600-MHz NMR spectrometer. Thirty-two transients were acquired for each of 200 t1 points. The sweep widths in ω1 and ω2 were 2500 and 5000 Hz, respectively. Before Fourier transformation, a 90°C shifted sine bell apodization and zero filling to 2048 data points were applied in each dimension. Volumes of the assigned (23) cross-peaks were measured by numerical integration using the program felix (Biosym Technologies, San Diego) and scaled according to the integral of a well resolved methyl resonance in the one-dimensional 1H NMR spectrum of DHFR, collected for each sample in parallel with the two-dimensional data.
Data Analysis.
The volume of each peak as a function of time of exposure to 2H2O was fit to a single exponential by the Levenberg–Marquardt nonlinear least-squares method (24). The errors of the measured rate constants were estimated by 10,000 iterations of Monte Carlo simulations, each randomly shifting the measured data points and repeating the least-square fitting, yielding a distribution of rate constants similar to that which would be obtained from multiple measurements (24). Only probes for which 68.3% of the simulated values (equivalent to ±1 standard deviation) were within 2-fold of the measured exchange rates were considered reliable. For the 23 probes in GroEL-bound DHFR deemed unreliable, bounds were placed on their exchange rates, beyond which they would not have been washed out during the refolding step. The random coil rate constant (krc) was derived by applying published primary sequence correction factors (25) to the exchange rate of the reference compound, poly-dl-alanine, experimentally determined in our buffer system (26).
RESULTS
DHFR Diluted from Denaturant Forms More Than One Folding Intermediate That Can Bind Rapidly to GroEL.
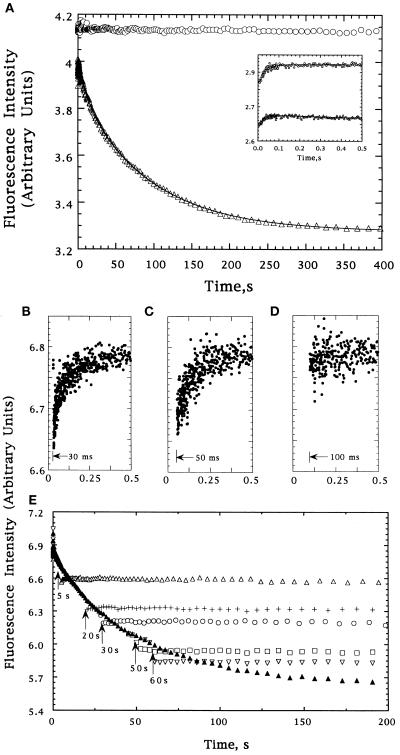
To determine which species in the kinetic refolding pathway of DHFR are recognized by GroEL, we examined the folding of DHFR by stopped-flow fluorescence spectroscopy in the presence and absence of GroEL (which contains no tryptophans). Fig. 1A shows the time dependence of DHFR tryptophan fluorescence intensity during refolding in the absence of GroEL at a final concentration of 1.2 μM. The fluorescence intensity increases over the first 0.1 sec, then decreases to the value expected for the native conformation by 400 sec. The data can be fit as a sum of three exponentials, a single rising phase with a relaxation time of 31 ± 6 msec (Fig. 1A Inset), and a decrease composed of two slower phases, with 12-sec and 91-sec relaxation times and relative amplitudes of 19% and 81%, respectively. When 1.2 μM GroEL (14-mer) is present in the refolding buffer, only a rising phase is observed, with a relaxation time of 26 ± 2 msec, similar to that observed in the absence of GroEL (Fig. 1A and Inset). This suggests that, at this concentration, DHFR binds to GroEL at a rate that is slower than the 31-msec folding reaction, but faster than the subsequent folding reactions, and that once bound to GroEL, DHFR tryptophan fluorescence ceases to change. Confirming this, when GroEL was injected into a solution of refolding DHFR at 30, 50, or 100 msec, the same increasing phase was observed (Fig. 1 B–D). By contrast, injections at later times (5, 20, 30, 50, and 60 sec) rapidly halted the decrease in fluorescence and, presumably, the folding of DHFR (Fig. 1E). At each time point, the fraction of DHFR that had already folded to the native state was apparently unaffected by the addition of GroEL.
Figure 1.
(A) Tryptophan fluorescence intensity changes of DHFR during refolding from 6 M urea in the absence (▵) or presence (○) of GroEL; the Inset shows the behavior for the first 0.5 sec. The data were fit to a sum of three exponentials using a nonlinear least-squares fitting program available from the SAS institute (Cary, NC). The lines reflecting these fits are shown. (B–E) Tryptophan fluorescence of refolding DHFR following addition of GroEL at various times after the initiation of folding. Injections after 30, 50, and 100 msec are shown independently in B–D, respectively. Injections after 5 (▵), 20 (+), 30 (○), 50 (□), and 60 (▿) sec are shown in E, as is a trace in the absence of GroEL (▴). The small differences between the intensity of the DHFR–GroEL complexes and the intensity of DHFR at the same folding times reflects the apparent cancellation of the decrease expected from the 2-fold dilution in the second mixing step by an increase due to addition of chaperone (50-fold excess by weight).
The observation that 25–30% of DHFR remains competent to bind to GroEL 60 sec after dilution from denaturant (Fig. 1E) implies that both slow folding phases are responsible for the depletion of species recognized by GroEL. If the GroEL binding-competent form were only removed by the 12-sec reaction, binding would not be observed after 55 sec when <1% of this form would remain. Likewise, if only the 91-sec reaction were responsible, 52% of the binding-competent DHFR should still be left to bind to the chaperone, contrary to the above observation. This result also rules out a sequential model because again only the 91-sec reaction would eliminate chaperone binding. Thus, the simplest explanation for the fluorescence intensity data is that the GroEL binding-competent form that persists after 100 msec in the absence of GroEL is actually a pair of intermediates that are formed by the 31-msec reaction and that fold through independent 12-sec and 91-sec channels either to a single native form or, perhaps, to two native or native-like forms that are not recognized by GroEL.
To test if DHFR can bind to GroEL prior to the 31-msec folding reaction, DHFR was diluted from denaturant to a final concentration of 1.0 μM into buffer containing 10 μM GroEL. Under these conditions, no time-dependent change in fluorescence intensity was observed. This suggests that, at these concentrations, the rate at which DHFR binds to GroEL is faster than the 31-msec folding reaction and that binding prevents subsequent changes in tryptophan fluorescence. Independent studies of DHFR refolding in the absence of GroEL showed a significant increase in fluorescence of the dye 1-anilinonapthalene-8-sulfonate during the dead time of mixing (data not shown), suggesting that an intermediate with substantial nonpolar surface is formed within 5 msec, consistent with the ability to bind to GroEL. Based on the observation that the binding reaction is faster than the 31-msec folding reaction at a GroEL concentration of 10 μM, and slower than the 31-msec folding reaction when GroEL is 1.2 μM, we estimate that the second-order rate constant for DHFR binding to GroEL is on the order of 1 × 107 M−1·sec−1. Concerning binding of the later intermediates of DHFR [e.g., when 1.2 μM GroEL is mixed with 1.0 μM DHFR which has been allowed to refold for 5–60 sec (Fig. 1E)], the lack of any observable binding kinetics suggests a second-order rate constant that is equal to or greater than that of the early intermediate. Thus, the fluorescence data indicate that GroEL is capable of binding rapidly not only to two distinct intermediates that are formed in a folding reaction with a time constant of 31 msec, but also to an early, burst phase folding intermediate of DHFR.
Measurement of Amide Proton Exchange in GroEL-Bound DHFR.
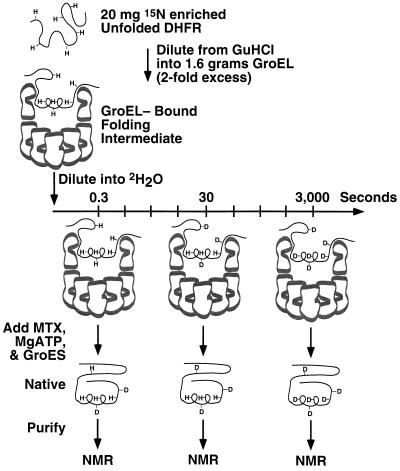
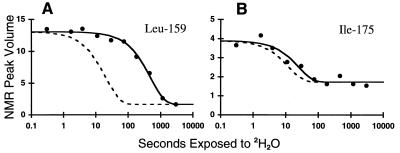
To probe the structure of DHFR while bound to GroEL, the degree and pattern of protection of its amide protons from deuterium exchange was measured. 15N-DHFR–GroEL binary complex was diluted into 2H2O; after each of 10 different periods of 2H2O exposure, ranging from 0.3 to 3000 sec, exchange was halted by refolding the DHFR to its native state by the addition of Mg·ATP, GroES, and the substrate analogue, MTX, which stabilizes native DHFR and prevents aggregation or rebinding (55) (see Fig. 2). Thus, instead of stopping exchange by acid-quenching, which led to wholesale precipitation, DHFR was converted to the native state, where it has been established that 65 of its 174 backbone amide protons are highly protected against exchange (23). The extent of exchange that had occurred prior to reaching the native, fully protected, state was quantified for these 65 “probes” by acquiring a two-dimensional NMR spectrum of each of the 10 samples of native DHFR, after chromatographic separation from GroEL and GroES. For each probe, least-squares fitting was used to determine the rate of decay of NMR peak volume (proportional to amide proton occupancy) as a function of time of exposure to 2H2O while bound to GroEL. This is shown for a moderately protected amide proton, that of Leu-159, and a relatively unprotected amide proton, that of Ile-175, in Fig. 3 A and B, respectively. The decay of each amide probe fit to a single exponential function with no systematic deviations, consistent with a single GroEL-bound species. Note that each of the 10 samples was prepared identically except for the time of exposure to 2H2O while bound to GroEL. Because the subsequent refolding step was identical for each of the 10 samples, exchange occurring during refolding would not affect the determination of the exchange rate constant of the species bound to GroEL, provided adequate proton occupancy remains for quantitation by NMR. This method of quenching exchange by refolding was sufficient for 42 of the 65 potential probes, while for 23 of the 65 probes refolding was not sufficiently rapid to allow reliable measurement of exchange that had occurred while bound to GroEL (see data analysis). Not surprisingly, the 23 probes washed out during refolding are among the most rapidly exchanging probes in native DHFR.
Figure 2.
Illustration of the procedure used to prepare samples for NMR analysis of amide exchange rates of GroEL-bound DHFR. The top ring of GroEL is shown as a cross-section. Amide protons protected from exchange are labeled as “H,” while those which exchange with solvent deuterons are labeled as “D.” After each of 10 time points, DHFR is refolded to the native state from which further exchange is negligible, separated from GroEL, and placed in an NMR spectrometer to measure the proton occupancy of each amide.
Figure 3.
Representative amide exchange data for residues Leu-159 (A) and Ile-175 (B). NMR peak volumes are plotted as a function of time of exposure of the GroEL-bound folding intermediate to 2H2O. The time scale is logarithmic. The solid lines are the result of nonlinear least-squares fitting of the data to a single exponential plus a constant. The dashed lines indicate the exchange rates calculated for the same amide in a random coil environment. Protection factors, defined by the ratio of the two rate constants, are 24 for Leu-159 and 2 for Ile-175.
Amide Protons in GroEL-Bound DHFR Corresponding to the Central β-Sheet Region of Native DHFR Exhibit Selective Protection Against Exchange.
Protection factors, defined as the ratio of the rate constant for exchange in a random coil of the same sequence (krc) to that measured in the GroEL-bound state (kobs), were assigned to each amide probe. Protection factors of the 42 measurable probes are listed in Table 1, along with upper bounds calculated for the 23 probes washed out during the refolding step. The protection factors of GroEL-bound DHFR range from 2 to 54. Most of the well protected amides are those of residues which in native DHFR are located in the central β-sheet. Overall, 18 of the 35 measurable probes in the β-sheet region have protection factors >10; another 11 have protection factors between 5 and 10 (Fig. 4 A and B), suggesting that this structure is likely present in GroEL-bound DHFR. The generally modest protection from exchange resembles that of other equilibrium and kinetic folding intermediates studied in the absence of chaperones (30–35). Our interpretation of the structure of GroEL-bound DHFR in terms of protection factors is based on the assumption that exchange occurs via an EX2 mechanism, in which the unprotected rate of exchange (given by krc) is much slower than the rate of reprotection of transiently exposed amide protons. If this assumption is not valid, the conclusion that GroEL-bound DHFR contains elements of structure would not change; however, the stability of such structure could not be described in terms of protection factors.
Table 1.
Protection factors (krc/kobs) of backbone amides measured for DHFR bound to GroEL and for native DHFR–MTX
| Residue | GroEL bound, krc/kobs | Native state, krc/kobs × 10−3 |
|---|---|---|
| N5 | <5 | 13,000 (12) |
| C6 | <11 | 32,000 (9) |
| I7 | 14 (24) | 39,000 (44) |
| V8 | 12 (12) | 5,300 (42) |
| A9 | 20 (30) | 190 (13) |
| V10 | 10 (15) | 5,700 (26) |
| S11 | <5 | 8,400 (11) |
| M14 | <5 | 260 (12) |
| G15 | <5 | 480 (13) |
| I16 | <5 | 810 (13) |
| G17 | <5 | 88 (14) |
| W24 | 10 (22) | 64 (13) |
| F31 | <5 | 36 (13) |
| R32 | <5 | 1,900 (93) |
| Y33 | <5 | 66 (20) |
| F34 | 16 (20) | 7,000 (15) |
| Q35 | <5 | 4,100 (9) |
| R36 | <5 | 2,000 (19) |
| M37 | <5 | 4,200 (10) |
| T38 | <5 | 6,900 (10) |
| T39 | <5 | 4,300 (7) |
| N48 | <7 | 390 (16) |
| L49 | 6 (32) | 11,000 (22) |
| V50 | 5 (17) | 16,000 (75) |
| I51 | 9 (19) | 15,000 (67) |
| M52 | 9 (40) | 40,000 (47) |
| F58 | 7 (26) | 14,000 (31) |
| S59 | <5 | 9,200 (7) |
| I60 | 6 (37) | 17,000 (31) |
| N64 | <7 | 440 (9) |
| I71 | 4 (20) | 7,500 (22) |
| L73 | 10 (44) | 52,000 (54) |
| V74 | 6 (13) | 21,000 (10) |
| L75 | 2 (23) | 7,700 (36) |
| S76 | <5 | 390 (6) |
| L97 | 13 (16) | 890 (8) |
| M111 | 12 (14) | 290 (26) |
| V112 | 33 (11) | 31 (9) |
| W113 | 40 (9) | 48,000 (10) |
| I114 | 54 (13) | 8,800 (55) |
| V115 | 27 (11) | 2,300 (26) |
| G116 | <5 | 170 (10) |
| A124 | <5 | 77 (8) |
| M125 | <5 | 83 (9) |
| K132 | 5 (22) | 2,500 (11) |
| L133 | 9 (20) | 11,000 (34) |
| F134 | 12 (12) | 41,000 (65) |
| V135 | 11 (11) | 35,000 (65) |
| T136 | 14 (14) | 110,000 (10) |
| R137 | 34 (19) | 330,000 (10) |
| I138 | 17 (10) | 53,000 (10) |
| M139 | 14 (25) | 62 (12) |
| Q140 | 8 (33) | 260 (7) |
| F148 | 26 (28) | 2,300 (8) |
| Y156 | 19 (11) | 320 (18) |
| K157 | 22 (20) | 2,600 (5) |
| L159 | 24 (9) | 1,100 (8) |
| E172 | <5 | 810 (42) |
| I175 | 2 (29) | 16 (10) |
| Y177 | 9 (29) | 10,000 (11) |
| K178 | 5 (22) | 16,000 (15) |
| F179 | 4 (25) | 7,600 (10) |
| E180 | 4 (24) | 15,000 (34) |
| V181 | 5 (13) | 150 (10) |
| Y182 | 12 (13) | 1,200 (11) |
| W24-Hɛ | <5 | 180 (10) |
| W57-Hɛ | <5 | 89 (7) |
| W113-Hɛ | 48 (20) | 170,000 (19) |
The last three lines show protection factors for the indole side chain NH protons of the three tryptophans in DHFR. For each protection factor, the error (parentheses) represents 1 SD, as a percentage of the listed value, estimated by Monte Carlo simulation (24). For the probes washed out during refolding, only upper bounds are listed. A protection factor of 5 implies that the amide is hydrogen bonded or otherwise protected from exchange 80% of the time; a protection factor of 10 implies 90% of the time.
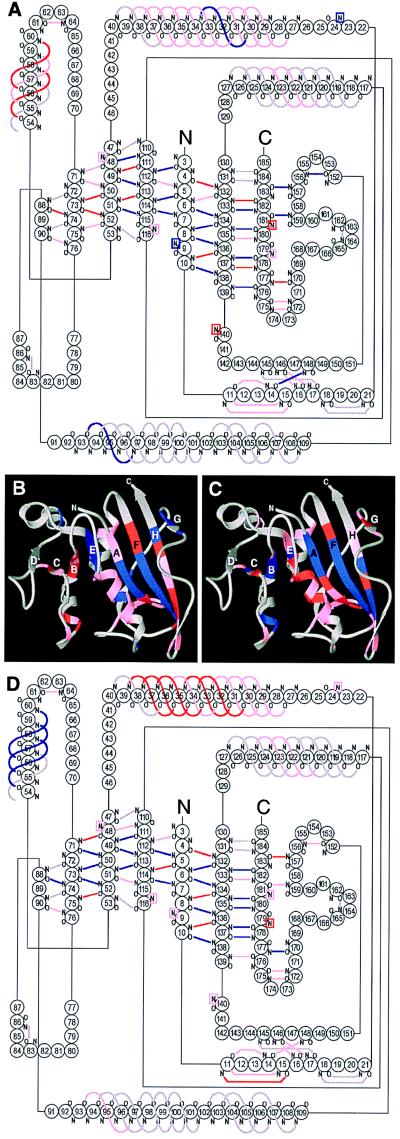
Figure 4.
Location of protected amides in GroEL-bound and native DHFR. (A) Diagram of hydrogen bonds in DHFR, colored according to the degree of protection in the GroEL-bound state. Blue represents protection factors >10, red between 5 and 10, pink <5 or unmeasurable, and gray hydrogen bonds that are inferred from the crystal structures but not observed by NMR. Probes that do not form hydrogen bonds directly with backbone or side chain acceptors are shown in boxes whose color reflects the degree of protection. Hydrogen bonds were identified from the x-ray structures of DHFR (27, 28) using the program hbplus (29). (B) Ribbon diagram of native DHFR (28), displaying the protection factors of the GroEL-bound state, colored as in A. (C and D) Ribbon (C) and hydrogen bond diagrams (D) displaying the protection factors measured for native DHFR. Blue represents protection factors >9 × 106, red between 2.5 × 106 and 9 × 106, and pink <2.5 × 106.
For comparison, amide exchange rates were also measured for native DHFR–MTX in the absence of GroEL by acquiring a series of heteronuclear single quantum coherence NMR spectra on a sample newly transferred to 99.9% 2H2O and following the exponential decrease in proton occupancy of each of the amide probes. Native DHFR is considerably more resistant to exchange than GroEL-bound DHFR, with protection factors of the 65 measurable probes ranging from 1.6 × 104 to 3.3 × 108 (see Table 1). Nevertheless, the pattern of protection resembles that of GroEL-bound DHFR, with the most protected amide protons located in the central 8-stranded parallel β-sheet (Fig. 4 C and D). The similarity is striking, especially considering that the data for native DHFR was collected in the presence of MTX, which was necessary to prevent aggregation.
A precise delineation of the structure of the nonnative state cannot be achieved by measurement of amide exchange by NMR because this technique is limited to probes that are protected from exchange in the native state, and because hydrogen bond acceptors are not specifically identified. This problem, however, can be addressed by examining the pattern of protection of a large set of probes (Fig. 4). While it cannot be excluded that the protection observed for GroEL-bound DHFR results from an alternative structure unrelated to the native one, the pattern of protection is explained most easily by the presence of a subset of the secondary structure elements found in native DHFR. Considering that the strands of the central β-sheet of native DHFR are derived from distant parts of the primary sequence, including both termini (Fig. 4), the presence of this central structure would imply that the global topology of GroEL-bound DHFR is native-like.
We considered whether these results could have been produced by a small population of fully native molecules in the sample of GroEL-bound DHFR. Such molecules, however, would have remained fully protonated even at the longest time point (3000 sec) and their amides would not have contributed to an exponential decrease of proton occupancy. Comparison of the occupancy at the longest time point with NMR data of a fully protonated control sample revealed that the entire population had reached equilibrium with the solvent by this time, indicating that no significant population could have been fully native. Conversely, any completely unfolded molecules would have exchanged at the random-coil rate and would have produced a second (faster) phase in the decay of amide proton occupancy as a function of 2H2O exposure time. This was not observed. Therefore, exchange rates measured at GroEL indicate that neither the fully folded nor fully unfolded states of DHFR are present.
DISCUSSION
The NMR analysis presented here offers a detailed view of the moderately stable conformation of GroEL-bound DHFR, which appears to possess both a core of native-like secondary structure and a native-like global topology. Our stopped-flow studies observed that GroEL can bind rapidly to DHFR both before and after the 31-msec folding phase detected by fluorescence. Other refolding studies (36–42) have shown that many proteins, including the structurally homologous E. coli DHFR (35, 43–45), can acquire substantial secondary structure and early tertiary contacts on this time scale. This suggests that GroEL can rapidly bind to folding intermediates that are already partly structured. The complex studied by NMR was formed at sufficiently high concentration that binding was likely complete before the 31-msec folding reaction. It is possible, however, that the structure observed by NMR does not correspond to the burst-phase intermediate of DHFR, because the length of time needed for concentration before exposure to 2H2O may allow the kinetic intermediate to equilibrate to the state with highest affinity for GroEL. Given the observation that later intermediates, with presumably greater structure, can also bind to GroEL with equal or greater rate constants, it is possible that these later intermediates have greater affinity for GroEL. Furthermore, some proteins, such as barnase (46–48), can fold while bound to GroEL, suggesting that partial folding of DHFR is possible even though human DHFR clearly cannot fold to native form while bound to GroEL. Recent studies of barnase (49) and α-lactalbumin (50) also conclude that for any given protein, the conformations recognized by GroEL are not unique, and suggest that GroEL can bind to different forms of the same protein with different affinities. For barnase it was reported that GroEL can affect the rates of interconversion between these forms (but see also ref. 51).
Currently, we can only speculate as to whether the structure of the GroEL-bound state of DHFR more closely resembles that of the kinetic folding intermediate formed in the 5-msec burst phase or that of one of the subsequent folding intermediates. The resolution of the stopped-flow fluorescence study is not sufficient to answer such a question, and in the absence of GroEL, human DHFR is too aggregation-prone to address this by other means. Because the exchange rates measured in the NMR study are consistent with the presence of a single GroEL-bound species, it seems likely that different kinetic folding intermediates in solution acquire a common conformation, perhaps that of the burst phase intermediate, once equilibrium is reached when bound to GroEL. It is also possible that more than one folding intermediate may be bound to GroEL at equilibrium, but may have the same conformation with respect to the measurable NMR probes.
DHFR seems to offer a particularly valuable system for studying recognition, considering that the E. coli enzyme, which is far less prone to aggregation during refolding, fails to be recognized by GroEL. E. coli DHFR shares a nearly identical fold to the human enzyme but only 32% sequence identity. GroEL apparently recognizes features exposed during the folding of human DHFR that are not presented by the E. coli enzyme. A recent study of Frieden and coworkers (52) has shown that insertion of amino acids into surface loops of the E. coli enzyme results in stable binding of nonnative forms to GroEL. Presumably, the mutants are altered in such a way that kinetically trapped forms exposing hydrophobic surfaces, perhaps similar to those in the human protein, are now recognized by GroEL. Alternatively, the altered loops themselves may be involved in binding. Unfortunately, the present study has not identified sites on human DHFR that interact with GroEL, perhaps because the interacting sites lack NMR probes.
As expected, our stopped-flow studies showed that binding of human DHFR by GroEL prevented the formation of aggregates of DHFR, which occurs in the absence of GroEL (data not shown). We conclude that during protein biogenesis, and presumably also during heat shock, GroEL captures structured folding intermediates before they can aggregate, presumably through exposed hydrophobic surfaces. The observation that significant structure is retained in folding intermediates upon binding to GroEL may reflect the critical ability of GroEL to bind rapidly to aggregation-prone folding intermediates that are already partly structured. Binding may act not only to prevent aggregation but also to lift proteins out of kinetic “traps” (15, 53, 54), committing them to productive folding in the sequestered central channel by the subsequent action of GroES and ATP.
Acknowledgments
We thank M. Cocco, M. Jacobs, K. MacKenzie, and J. Tolman for assistance with NMR; F. Richards for discussions; W. Fenton for critical reading; and M. Jacobs for assistance with the Monte Carlo simulations and suggestions throughout. This work was supported by grants from the National Institutes of Health.
Footnotes
Abbreviations: DHFR, dihydrofolate reductase; MTX, methotrexate.
References
- 1.Weissman J S, Hohl C M, Kovalenko O, Kashi Y, Chen S, Braig K, Saibil H R, Fenton W A, Horwich A L. Cell. 1995;83:577–587. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 2.Mayhew M, da Silva A C, Martin J, Erdjument-Bromage H, Tempst P, Hartl F U. Nature (London) 1996;379:420–426. doi: 10.1038/379420a0. [DOI] [PubMed] [Google Scholar]
- 3.Weissman J S, Rye H S, Fenton W A, Beechem J M, Horwich A L. Cell. 1996;84:481–490. doi: 10.1016/s0092-8674(00)81293-3. [DOI] [PubMed] [Google Scholar]
- 4.Braig K, Otwinowski Z, Hegde R, Boisvert D C, Joachimiak A, Horwich A L, Sigler P B. Nature (London) 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- 5.Landry S J, Gierasch L M. Annu Rev Biophys Biomol Struct. 1994;23:645–669. doi: 10.1146/annurev.bb.23.060194.003241. [DOI] [PubMed] [Google Scholar]
- 6.Fenton W A, Kashi Y, Furtak K, Horwich A L. Nature (London) 1994;371:614–619. doi: 10.1038/371614a0. [DOI] [PubMed] [Google Scholar]
- 7.Itzhaki L S, Otzen D E, Fersht A R. Biochemistry. 1995;34:14581–14587. doi: 10.1021/bi00044a037. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Roseman A M, Hunter A S, Wood S P, Burston S G, Ranson N A, Clarke A R, Saibil H R. Nature (London) 1994;371:261–264. doi: 10.1038/371261a0. [DOI] [PubMed] [Google Scholar]
- 9.Hunt J F, Weaver A J, Landry S J, Gierasch L, Deisenhofer J. Nature (London) 1996;379:37–45. doi: 10.1038/379037a0. [DOI] [PubMed] [Google Scholar]
- 10.Mande S C, Mehra V, Bloom B R, Hol W G. Science. 1996;271:203–207. doi: 10.1126/science.271.5246.203. [DOI] [PubMed] [Google Scholar]
- 11.Saibil H. Structure (London) 1996;4:1–4. doi: 10.1016/s0969-2126(96)00002-0. [DOI] [PubMed] [Google Scholar]
- 12.Roseman A M, Chen S, White H, Braig K, Saibil H R. Cell. 1996;87:241–251. doi: 10.1016/s0092-8674(00)81342-2. [DOI] [PubMed] [Google Scholar]
- 13.Todd M J, Viitanen P V, Lorimer G H. Science. 1994;265:659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- 14.Weissman J S, Kashi Y, Fenton W A, Horwich A L. Cell. 1994;78:693–702. doi: 10.1016/0092-8674(94)90533-9. [DOI] [PubMed] [Google Scholar]
- 15.Ranson N A, Dunster N J, Burston S G, Clarke A R. J Mol Biol. 1995;250:581–586. doi: 10.1006/jmbi.1995.0399. [DOI] [PubMed] [Google Scholar]
- 16.Taguchi H, Yoshida M. FEBS Lett. 1995;359:195–198. doi: 10.1016/0014-5793(95)00041-7. [DOI] [PubMed] [Google Scholar]
- 17.Smith K E, Fisher M T. J Biol Chem. 1995;270:21517–21523. doi: 10.1074/jbc.270.37.21517. [DOI] [PubMed] [Google Scholar]
- 18.Burston S G, Weissman J S, Farr G W, Fenton W A, Horwich A L. Nature (London) 1996;383:96–99. doi: 10.1038/383096a0. [DOI] [PubMed] [Google Scholar]
- 19.Zahn R, Spitzfaden C, Ottiger M, Wüthrich K, Plückthun A. Nature (London) 1994;368:261–265. doi: 10.1038/368261a0. [DOI] [PubMed] [Google Scholar]
- 20.Robinson C V, Gross M, Eyles S J, Ewbank J J, Mayhew M, Hartl F U, Dobson C M, Radford S E. Nature (London) 1994;372:646–651. doi: 10.1038/372646a0. [DOI] [PubMed] [Google Scholar]
- 21.Gervasoni P, Staudenmann W, James P, Gehrig P, Plückthun A. Proc Natl Acad Sci USA. 1996;93:12189–12194. doi: 10.1073/pnas.93.22.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landry S J, Gierasch L M. Biochemistry. 1991;30:7359–7352. doi: 10.1021/bi00244a001. [DOI] [PubMed] [Google Scholar]
- 23.Stockman B J, Nirmala N R, Wagner G, Delcamp T J, DeYarman M T, Freisheim J H. Biochemistry. 1992;31:218–229. doi: 10.1021/bi00116a031. [DOI] [PubMed] [Google Scholar]
- 24.Press W H, Flannery B P, Teukolsky S A, Vetterling W T. Numerical Recipes. Cambridge: Cambridge Univ. Press; 1986. pp. 521–538. [Google Scholar]
- 25.Bai Y, Milne J S, Mayne L, Englander S W. Proteins. 1993;17:75–86. doi: 10.1002/prot.340170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Englander J J, Calhoun D B, Englander S W. Anal Biochem. 1979;92:517–524. doi: 10.1016/0003-2697(79)90693-6. [DOI] [PubMed] [Google Scholar]
- 27.Davies J F, Delcamp T J, Predergast N J, Ashford V A, Freisheim J H, Kraut J. Biochemistry. 1990;29:9467–9479. doi: 10.1021/bi00492a021. [DOI] [PubMed] [Google Scholar]
- 28.Oefner C, D’arcy A, Winkler F K. Eur J Biochem. 1988;174:377–385. doi: 10.1111/j.1432-1033.1988.tb14108.x. [DOI] [PubMed] [Google Scholar]
- 29.McDonald I K, Thornton J M. J Mol Biol. 1994;238:777–793. doi: 10.1006/jmbi.1994.1334. [DOI] [PubMed] [Google Scholar]
- 30.Udgaonkar J B, Baldwin R L. Proc Natl Acad Sci USA. 1990;87:8197–8201. doi: 10.1073/pnas.87.21.8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeng M-F, Englander S W, Elöve G A, Wand A J, Roder H. Biochemistry. 1990;29:10433–10437. doi: 10.1021/bi00498a001. [DOI] [PubMed] [Google Scholar]
- 32.Hughson F M, Wright P E, Baldwin R L. Science. 1990;249:1544–1548. doi: 10.1126/science.2218495. [DOI] [PubMed] [Google Scholar]
- 33.Lu J, Dahlquist F W. Biochemistry. 1992;31:4749–4756. doi: 10.1021/bi00135a002. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs M D, Fox R O. Proc Natl Acad Sci USA. 1994;91:449–453. doi: 10.1073/pnas.91.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones B E, Matthews C R. Protein Sci. 1995;4:167–177. doi: 10.1002/pro.5560040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udgaonkar J B, Baldwin R L. Nature (London) 1988;335:694–699. doi: 10.1038/335694a0. [DOI] [PubMed] [Google Scholar]
- 37.Roder H, Elöve G A, Englander S W. Nature (London) 1988;335:700–704. doi: 10.1038/335700a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bycroft M, Matouschek A, Kellis J T, Jr, Serrano L, Fersht A R. Nature (London) 1990;346:488–490. doi: 10.1038/346488a0. [DOI] [PubMed] [Google Scholar]
- 39.Briggs M S, Roder H. Proc Natl Acad Sci USA. 1992;89:2017–2021. doi: 10.1073/pnas.89.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullins L S, Pace C N, Raushel F M. Biochemistry. 1993;32:6152–6156. doi: 10.1021/bi00075a006. [DOI] [PubMed] [Google Scholar]
- 41.Jennings P A, Wright P E. Science. 1993;262:892–896. doi: 10.1126/science.8235610. [DOI] [PubMed] [Google Scholar]
- 42.Ptitsyn O B. Curr Opin Struct Biol. 1995;5:74–78. doi: 10.1016/0959-440x(95)80011-o. [DOI] [PubMed] [Google Scholar]
- 43.Kuwajima K, Garvey E P, Finn B E, Matthews C R, Sugai S. Biochemistry. 1991;30:7693–7703. doi: 10.1021/bi00245a005. [DOI] [PubMed] [Google Scholar]
- 44.Jones B E, Jennings P A, Pierre R A, Matthews C R. Biochemistry. 1994;33:15250–15258. doi: 10.1021/bi00255a005. [DOI] [PubMed] [Google Scholar]
- 45.Jones B E, Beechem J M, Matthews C R. Biochemistry. 1995;34:1867–1877. doi: 10.1021/bi00006a007. [DOI] [PubMed] [Google Scholar]
- 46.Gray T E, Eder J, Bycroft M, Day A G, Fersht A R. EMBO J. 1993;12:4145–4150. doi: 10.1002/j.1460-2075.1993.tb06098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray T E, Fersht A R. J Mol Biol. 1993;232:1197–1207. doi: 10.1006/jmbi.1993.1471. [DOI] [PubMed] [Google Scholar]
- 48.Corrales F J, Fersht A R. Proc Natl Acad Sci USA. 1995;92:5326–5330. doi: 10.1073/pnas.92.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zahn R, Perrett S, Fersht A R. J Mol Biol. 1996;261:43–61. doi: 10.1006/jmbi.1996.0440. [DOI] [PubMed] [Google Scholar]
- 50.Katsumata K, Okazaki A, Kuwajima K. J Mol Biol. 1996;258:827–838. doi: 10.1006/jmbi.1996.0290. [DOI] [PubMed] [Google Scholar]
- 51.Walter S, Lorimer G H, Schmid F X. Proc Natl Acad Sci USA. 1996;93:9425–9430. doi: 10.1073/pnas.93.18.9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark A C, Hugo E, Frieden C. Biochemistry. 1996;35:5893–5901. doi: 10.1021/bi953051v. [DOI] [PubMed] [Google Scholar]
- 53.Jackson G S, Staniforth R A, Halsall D J, Atkinson T, Holbrook J J, Clarke A R, Burston S G. Biochemistry. 1993;32:2554–2563. doi: 10.1021/bi00061a013. [DOI] [PubMed] [Google Scholar]
- 54.Todd M J, Lorimer G H, Thirumalai D. Proc Natl Acad Sci USA. 1996;93:4030–4035. doi: 10.1073/pnas.93.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viitanen P V, Donaldson G K, Lorimer G H, Lubben T H, Gatenby A A. Biochemistry. 1991;30:9716–9723. doi: 10.1021/bi00104a021. [DOI] [PubMed] [Google Scholar]