Abstract
T lymphocyte activation is associated with nitric oxide (NO) production that plays an essential role in multiple T cell functions. NO acts as a messenger, activating soluble guanyl cyclase and participating in the transduction signaling pathways involving cyclic GMP. NO modulates mitochondrial events that are involved in apoptosis and regulates mitochondrial membrane potential and mitochondrial biogenesis in many cell types, including lymphocytes. Mitochondrial hyperpolarization (MHP), an early and reversible event during both T lymphocyte activation and apoptosis, is regulated by NO. Here, we discuss recent evidence that NO-induced MHP represents a molecular switch in multiple T cell signaling pathways. Overproduction of NO in systemic lupus erythematosus (SLE) induces mitochondrial biogenesis and alters Ca2+ signaling. Thus, while NO plays a physiological role in lymphocyte cell signaling, its overproduction may disturb normal T cell function, contributing to the pathogenesis of autoimmunity.
Keywords: Nitric oxide, apoptosis, mitochondrial hyperpolarization, mitochondrial biogenesis, calcium
Introduction
The mitochondrion, the site of oxidative phosphorylation, has long been identified as a source of energy and cell survival [1, 2]. The electrochemical gradient across the inner mitochondrial membrane is maintained by the electron transport chain (ETC) and Δψm (negative inside and positive outside) [3]. The Δψm is subject to regulation by an oxidation-reduction equilibrium of ROI, pyridine nucleotides (NADH/NAD + NADPH/NADP) and GSH levels [4]. Controlled levels of ROI modulate various aspects of cellular function and are necessary for signal transduction pathways, including those mediating T-cell activation and apoptosis [5] (Fig. 1). Elevation of Δψm or mitochondrial hyperpolarization (MHP) was discovered in our laboratory [6]. MHP in T cells is facilitated by depletion of GSH and NADPH triggered by over-expression of the PPP enzyme transaldolase (TAL) [6, 7]. Transient MHP is an early event preceding caspase activation, phosphatidylserine (PS) externalization, and disruption of Δψm in Fas- [6] and H2O2-induced apoptosis of Jurkat human leukemia T cells and normal human peripheral blood lymphocytes (PBL) [8]. These observations were widely confirmed and extended to other apoptosis pathways [9, 10, 11, 12, 13, 14]. Transient MHP is also triggered by activation of T cells by Con A [6] and CD3/CD28 costimulation [15] via ROI- and Ca2+-dependent production of NO [16]. Thus, MHP represents an early and reversible switch not exclusively associated with apoptosis. In contrast, as recently revealed in our laboratory, T cells of SLE patients show persistent MHP and ATP depletion which cause abnormal T-cell death, shifting susceptibility from apoptosis to necrosis [15]. ATP depletion in lupus T cells was recently confirmed by Krishnan et al [17]. Increased necrosis rates could play a central role in enhanced inflammatory responses in SLE [18]. Necrotic cell death stimulates macrophages [19] and dendritic cells [20] and leads to inflammation [15, 18, 21] and development of murine SLE [22]. We proposed that increased release of necrotic materials from T cells would activate macrophages and dendritic cells (DCs) and enhance their capacity to produce NO and IFN-α̣ in SLE [18]. Indeed, DCs exposed to necrotic, but not apoptotic, cells induce lupus like-disease in MRL mice and accelerate the disease of MRL/lpr mice [22]. These data suggest that principal mitochondrial functions are involved in T cell activation. Nitric oxide (NO) is produced by T cells during activation and it regulates T cell signal transduction [1, 16, 18]. NO affects both crucial functions of the mitochondrion, the generation of energy and the control of cell death through modulating production of reactive oxygen intermediates and of ATP. This review will focus on the central role of NO in mitochodrial control of activation and cell death pathway selection in human T lymphocytes.
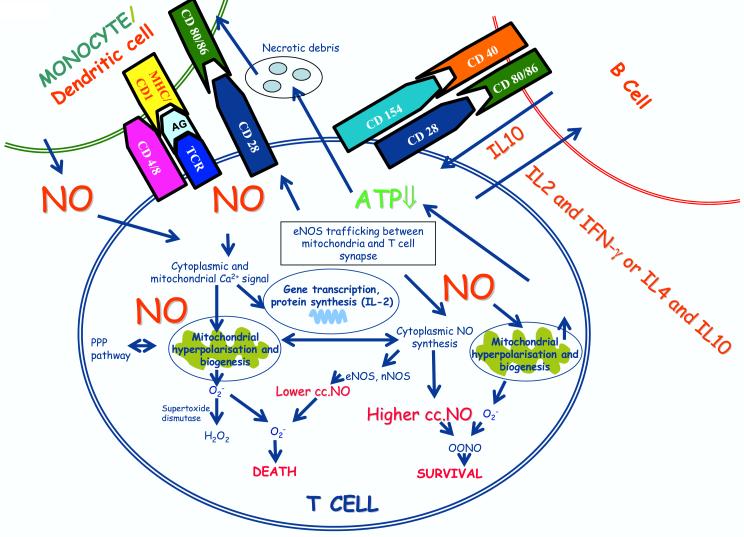
Legend to Fig. 1.
Schematic diagram of T cell activation, NO production, and mitochondrial hyperpolarization. NO is produced in the cytosol, the mitochondrial membrane, and at the immunological synapse of T cells. Localized NO production has been linked to targeting of eNOS to the outer mitochondrial membrane and to the T-cell synapse. NO decompose superoxide via ONOO- formation, thereby limiting the steady state concentrations of this oxygen-derived free radical. Furthermore, the interaction of superoxide with NO will result the elimination of superoxide-derived H2O2. Indeed, these types of interactions would likely extend the life of the T-cell by providing a NO-dependent mechanism to reduce both superoxide and H2O2. NO can freely diffuse through biological membranes and NO produced by other cells, such as monocytes and dendritic cells can affect T-cell activation. NO regulates many step of T cell activation, production of cytokines, such as IL2, and mitochondrial hyperpolarization and mitochondrial bioegenesis. Mitochondrial hyperpolarization is associated with depletion of ATP which predisposes T cells to necrosis. In turn, necrotic materials released from T cells activate monocytes and dendritic cells. Altered T-cell cytokine production also influences activation of B cells.
TCR activation, Ca2+ signaling
Engagement of the T cell antigen receptor (TCR) leads to activation of multiple protein tyrosine and serine kinases resulting in phosphorylation of intracellular substrates. The subsequent hydrolysis of phospholipids and elevation of cytoplasmic Ca2+ ultimately leads to clonal expansion of antigen-specific T cells [23]. An increase in cytoplasmic Ca2+ is essential for and is an early marker of T cell activation. The TCR is associated with a multimeric receptor module, comprised of the CD3 γδε and TCR ζ-chains. The cytoplasmic domain of CD3 and ζ-chains contain a common motif termed IgR family tyrosine based activation motif (ITAM) [24, 25]. Each ζ homodimer possesses 3 ITAMS, whereas other CD3 chains each contain 1 ITAM.
The most proximal biochemical event following T cell activation is the activation of protein tyrosine kinases. The interaction of the T cell specific CD4 or CD8 with P56lck, a member of the src family of PTKs, leads to an increase in P56lck kinase autophosphorylation, leading to phosphorylation of ITAM. Overexpression of an activated form of P56lck enhances TCR mediated activation. A signaling mutant of the Jurkat T cell line JaCam1, that fails to demonstrate an elevation of the intracellular Ca2+ upon TCR signaling has been shown to lack P56lck protein [26]. Phosphorylation of ITAM motifs are required for Src homology 2 domain-mediated binding by ζ-associated protein (ZAP70). ZAP 70 is activated through phosphorylation by P56lck. Activated ZAP-70 phosphorylates LAT adaptor protein, followed by the direct binding of LAT to phospholipase C-γ1.
Activation of the TCR by monoclonal antibodies, mitogenic lectins, or specific antigen on the surface of appropriate antigen-presenting cells elicits substantial, sustained increases in IP3 and in the concentration of cytoplasmic free Ca2+ within T lymphocytes. Phospholipase C-γ1 mediates IP3 dependent Ca2+ signal in T cells, similarly to B cells [27, 28]. Ca2+ serves as an intracellular messenger regulating different signaling pathways that leads to activation of protein kinase C (PKC), calmodulin and calmodulin dependent protein kinases, as well as calcineurin and translocation of the nuclear factor of activated T cells (NFAT) into the nucleus [29].
Activation of T cells through the TCR initiates rapid increase in cytoplasmic and mitochondrial Ca2+ levels (within 5−10 min), followed by a plateau phase, lasting at least 48 h [27]. Inhibition of the endoplasmic reticulum Ca2+ ATP-ase by thapsigargin results in emptying of the intracellular Ca2+ stores and capacitative Ca2+ influx from extracellular space.
Mitochondrial Ca2+ and mitochondrial transmembrane potential
Isolated mitochondria increase their rate of ATP production several-fold in response to increasing concentrations of ADP and Pi with unlimited supply of substrate and oxygen [30]. In addition to ADP, mitochondrial Ca2+ is another important regulator of mitochondrial ATP production. Exposure of isolated mitochondria to artificially created Ca2+ pulses, like those seen in the cytosol of the cells led to the discovery of mitochondrial Ca2+ uptake. Mitochondria take up Ca2+ primarily through an uniporter, whose molecular nature is still controversial. This might act like a channel, opening with increased probability once the local Ca2+ rises [31]. The major targets of the mitochondrial Ca2+ import pathway are the dehydrogenases of the citric acid cycle, as the rate limiting enzymes are all upregulated by Ca2+ dependent processes. In addition intramitochondrial Ca2+ activates the ETC, adenine nucleotide translocator (ANT) and F1F0-ATP-ase [32] thus oxidative phosphorylation is rapidly stimulated by pulses of Ca2+. The continuous charging of mitochondria with Ca2+ will sustain the activation state of the mitochondrial dehydrogenases and may be important in sustaining mitochondrial energy production [Fig 1.]. Mitochondrial Ca2+ uptake is primarily driven by the electrochemical potential gradient and a relatively low intramitochondrial Ca2+ concentration. Typically, mitochondrial Ca2+ uptake follows the cytosolic signal. Since re-equilibration of mitochondrial Ca2+ is relatively slow, mitochondria effectively integrate cytosolic Ca2+ signals over time [33].
The process of mitochondrial respiration establishes a large potential gradient across the inner mitochondrial membrane, the mitochondrial membrane potential (Δψm), generally estimated to be in the order of 150−200mV, negative inside [1]. The biochemical mechanism of oxidative phosphorylation involves two main steps: the transduction of chemical redox potentials into an electrochemical H+ gradient across the inner mitochondrial membrane and the ATP synthesis by the H+ driven molecular rotor of F0F1-ATP-ase. While the principal function of the mitochondrial potential is clearly to drive ATP synthesis, it also provides the major mechanism to handle Ca2+.
The mitochondrial membrane potential lies at the heart of all the major bioenergetic functions of the mitochondrion, from the synthesis of ATP to accumulation of Ca2+. The collapse of Δψm as a response to pathological states, such as anoxia or as a response to disordered mitochondrial respiration, will limit mitochondrial Ca2+ uptake and may contribute to cellular pathophysiology [32,34]. Mitochondrial Ca2+ import is an electrogenic process, as the movement of Ca2+ is not countered by any other ion exchange and therefore acts like an inward current, tending to depolarize the mitochondrial membrane through increasing the permeability of the voltage-dependent anion channel (VDAC) [35]. This is observed as a small and transient depolarization of the mitochondrial membrane in response in the early phase of the Ca2+ flux. Although the activation of dehydrogenases stimulates mitochondrial respiration, leading to mitochondrial hyperpolarization (MHP) and increased ATP production, following the above mentioned initial depolarization in many cell types [1], Ca2+ influx by ionomycin or Ca2+ release from intracellular stores by thapsigargin alone failed to induce Δψm elevation in lymphocytes [16].
Role of nitric oxide in T cell activation, mitochondrial biogenesis and hyperpolarization
Nitric oxide is a diffusible, multifunctional, transcellular messenger that has been implicated in a numerous physiological and pathological conditions [36]. NO is synthesized from L-arginine by NO synthases (NOS). Three distinct cytoplasmic isoforms of NOS are known, including neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS) enzymes. nNOS and eNOS are expressed constitutively and generate NO for constitutive cell-signaling purposes, while iNOS releases NO in larger quantities during inflammatory or immunological defense reactions and is involved in host tissue damage [37]. The physiological role of NO in the maintenance of vascular tone, in synaptic transmission and in cellular defense is firmly established. Recent evidence indicates that NO regulates mitchondrial biogenesis. NO competes with molecular oxygen (O2) and reversibly inhibit cytochrome C oxidase, suggesting that this competitive interaction has a physiological role in the control of mitochondrial respiration [36].
Crosslinking of the TCR has been associated with elevation of the cytosolic Ca2+, ROI and nitric oxide (NO) production and mitochondrial hyperpolarization [16]. NO chelator C-PTIO (carboxy-2-phenyl-4,4,5,5-tetrametyl-imidazoline-1-oxyl-3-oxide) profoundly inhibited TCR crosslinking induced mitochondrial hyperpolarization, cytoplasmic and mitochondrial Ca2+ response, ROI production and NO levels, suggesting that TCR activation induced MHP is mediated by NO [16]. In addition, NO signal was required for CD3/CD28 induced Ca2+ fluxing, suggesting an essential role of NO in T cell activation. Western blot analysis revealed expression of eNOS and nNOS and absence of iNOS in human PBL. eNOS and nNOS protein levels were stimulated up to 15-fold by CD3/CD28 costimulation [16, 38]. Ibiza and colleagues recently showed that within minutes of binding to antigen, T cells produce NO via endothelial NO synthase (eNOS) [39]. NO promoted mitochondrial hyperpolarization, ATP depletion and relative resistance to apoptotic stimuli in astrocytes, lymphocytes and Jurkat cells [13, 40]. Although pretreatment of normal T cells with NO resulted in elevation of mitochondrial and cytoplasmic Ca2+ levels, Ca2+ by itself was insufficient to induce NO synthesis or mitochondrial hyperpolarization in lymphocytes [16, 39].
NO has been recognized as a key signal for mitochondrial biogenesis. This operates through the cGMP-dependent peroxisome proliferator-activating receptor γ coactivator-1α, a master regulator of mitochondrial biogenesis [41]. NO was shown to induce mitochondrial biogenesis in brown adipocytes, U937 and HeLa cells and human lymphocytes [42, 43]. Studies of T lymphocytes lacking eNOS will be important to establish an absolute requirement of this NOS isoform in T-cell activation.
T cell activation involves considerable reordering of cell surface and cytoplasmic components into an immunological synapse [44]. Synapse formation is dependent on TCR mediated signals that, in concert with costimulatory signals, cause the cellular polarization of the T cell cytoskeleton, membrane receptors and selected cytoplasmic signaling effectors towards the T cell – antigen presenting cell interface. Although not required for TCR signal initiation, synapse formation has been associated with the induction of T cell proliferation, cytokine production and lytic granule release. Quantitative subcellular analysis of NO production in T cell APC conjugates showed that local NO production was 2-fold higher at cell-cell contact than in the cytoplasm [39]. The localized production of NO by eNOS at the immunological synapse is a newly recognized checkpoint of T cell activation which leads to increased phosphorylation of CD3 ζ chain, ZAP-70, and extracellular signal-regulated kinases and increased IFN-γ synthesis, but reduced production of IL-2 [39] (Fig 1.).
In addition to the NO originating from the cytoplasm, now it is clear that mitochondrion has its own NO production. NO production by the co-oxidation of L-arginine and NADPH by O2 has been observed in mitochondrial membranes isolated from a series of mammalian organs. The responsible enzyme has been named initially mitochondrial nitric oxide synthase (mtNOS) referring to its intracellular localization, in contrast to nNOS, eNOS and iNOS, that were defined according to the cell type of origin [45]. The mitochondrial NOS isoenzyme is a constitutive protein of the mitochondrial outer membrane that generates NO in a Ca2+ dependent reaction. mtNOS activity shows very strong and exponential dependence on mitochondrial membrane potential in physiological potential range (150−200mV), which observation suggests that mtNOS is regulated by Δψm [46]. However, a distinct mNOS gene has not yet been cloned and its very existence is controversial. Recent findings suggest that mtNOS may correspond to eNOS selectively targeted to the outer mitochondrial membrane [47].
Mitochondrial oxidative stress
In most cell types, mitochondria appear to represent one of the mayor sources of generation of reactive oxygen intermediates (ROI), such as superoxide anion O2− [1] (Fig 1.). Formation of the superoxide anion in the mitochondrion occurs at the level of complex I and complex III of the ETC [48]. Although they are well known for their destructive effect on biomolecules, ROI are more and more accepted as necessary constituents in signaling pathways and modulators of responses in physiological and pathological conditions [49 ]. In T cells, it has been reported that the radical scavenger N-acetyl cysteine (NAC) inhibited the activation of NF-κB by phorbol 12-myristate 13-acetate, tumor necrosis factor-α, and interleukin-1, strongly supporting the idea that oxygen radicals are implicated in physiological activation processes [50, 51]. ROI species trigger several proximal and distal signaling pathways in T lymphocytes, affect the activities of transcription factors, and lead to expression of specific genes (Fig 1.). In Jurkat T cells ROI induce increases in protein tyrosine phosphorylation and activity of p56lck, ZAP-70, and protein kinase C as well as elevations in intracellular Ca2+ levels [52]. ROI are known to mediate the activation of NF-κB, but chronic exposure to ROI inhibits NF-κB phosphorylation and activation.
As it was mentioned above, increased intramitochondrial Ca2+ activates the ETC, thereafter it stimulates mitochondrial respiration and ROI production [1]. Superoxide is effectively converted to hydrogen peroxide (H2O2) by the mitochondrial superoxide dismutase (SOD) (Fig 1.). H2O2 has no unpaired electrons and, by itself, is not a ROI. H2O2 could leave the mitochondrion and act as a second messenger that can mediate gene transcription and cell proliferation. Induction of apoptosis or necrosis by H2O2 requires transformation into an ROI, e.g., OH−, through the Fenton reaction [53].
The role of NO in apoptosis has been widely investigated, and both pro and anti-apoptotic actions have been reported [54, 55, 56]. Fas-induced apoptosis was found to be associated with an early, concentration dependent NO production in Jurkat cells [57]. NO can induce apoptosis in a variety of cell lines including macrophages, thymocytes, T cells, myeloid cells. Low concentrations of NO may specifically inhibit cytochrome c oxidase, leading to ATP depletion [58]. A site for NO modulation of the apoptotic process that has received little attention is the controlled release of cytochrome c from the mitochondria [59]. The release of cytochrome C from mitochondria leads to activation of caspases 9 and 3, thus the proapoptotic effect of NO may be connected to its effect on mitochondrial respiration.
NO reacts rapidly with superoxide (O2−) to produce peroxynitrite (ONOO−), which may act as an oxidant itself, or it may isomerise to nitrate. Peroxynitrite reacts with protein and non protein thiols, tyrosine residues, unsaturated fatty acids, as well as DNA, and it was reported to cause Ca2+ efflux from liver mitochondria [60, 61, 62]. Addition of peroxynitrite to the mitochondria also causes opening of the permeability transition pore (PTP) and opening of this pore contributes to the aforementioned loss of cytochrome C from the mitochondrion. Furthermore peroxynitrite inhibits, or damages mitochondrial complexes and SOD (Fig 1.). ROS may cause lipid peroxidation and damage to cell membranes and to DNA, so that mitochondria represent not only a major source of ROS generation, but also a major target of ROS induced damage.
Mitochondrial hyperpolarization and increased NO production in sytemic lupus erythematosus (SLE)
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by production of antinuclear autoantibodies, clinical involvement in multiple organ systems and increased NO production in monocytes [43]. Lupus T cells exhibit persistent MHP or elevation of the mitochondrial transmembrane potential, increased ROI levels and ATP depletion [15]. T lymphocytes of patients with SLE showed increased spontaneous and decreased activation-induced apoptosis [63]. Upon T cell activation, cell death preferentially occurred via necrosis rather than apoptosis in patients with SLE. ATP levels and ATP/ADP ratios were profoundly diminished in lupus PBL, which accounts for predisposition to necrosis [64].
Persistent MHP has been associated with increased mitochondrial mass of T lymphocytes in patients with SLE [43]. Mitochondria can take up, store and release Ca2+, thereafter increased mitochondrial mass may account for altered Ca2 handling [2]. Although SLE T cells and normal T cells produce comparable amount of NO, lupus monocytes were found to produce a significantly higher amount of NO than normal monocytes [43, 65]. In comparison to control monocytes, lupus monocytes increased Δψm and Ca2+ of normal T cells after co-culture [43], suggesting that NO produced by monocytes is responsible for mitochondrial dysfunction and predisposition to necrosis by lupus T cells. Release of necrotic materials from T cells activates monocytes and dendritic cells which may operate as a positive feed-back loop sustaining a pro-inflammatory state in SLE [2, 18].
Baseline cytoplasmic Ca2+ levels are increased in SLE T cells. While the early phase of Ca2+ signal was enhanced, sustained elevation of CD3/CD28-induced Ca2+ signaling was markedly reduced in lupus T cells [43]. Rapamycin (RAPA), a macrolide immunosuppressant that has been shown to interfere with T cell activation events, on the course of spontaneous disease progression in the MRL/lpr mouse model of lupus. RAPA sensitive pathway ha been shown to regulate mitochondrial membrane potential in irradiated human breast cancer (MCF-7) cells [66]. RAPA significantly reduced or prevented many pathologic features of lupus normally seen in the MRL/lpr mouse [67]. RAPA treatment of patients with SLE normalized cytosolic and mitochondrial Ca2+ levels and T cell activation-induced rapid Ca2+ fluxing, without influencing MHP [68], indicating that increased Ca2+ fluxing is downstream or independent of MHP in the pathogenesis of T-cell dysfunction in SLE. Interestingly, the mammalian target of rapamycin (mTOR) may be activated by enhanced production of NO [69]. The effectiveness of RAPA in murine and human SLE suggest that mTOR is a potential sensor [70] and down-stream effector of MHP and activation of mTOR may precede T cell dysfunction and autoimmunity in SLE. These new data suggest that the clinically beneficial role of RAPA may be related to its selective effect on Ca2+ fluxing in SLE.
Acknowledgments
This work was supported by grants RO1 AI 48079, RO1 DK49221, and R21 AI 061066 from the National Institutes of Health and the Central New York Community Foundation.
Abbreviations used in this paper:
- ANT
adenine nucleotide translocator
- C-PTIO
carboxy-2-phenyl-4,4,5,5-tetrametyl-imidazoline-1-oxyl-3-oxide
- ETC
electron transport chain
- ITAM
IgR family tyrosine based activation motif
- LAT
linker for activation
- IP3
inositol-1,4,5-triphosphate
- PI3K
phosphatodylinositol 3-hydroxyl kinase
- ROI
reactive oxygen intermediate
- SOD
superoxide dismutase
- Δψm
Mitochondrial transmembrane potential
- MHP
Mitochondrial hyperpolarization
- NOS
NO synthase
- nNOS
neuronal NOS
- eNOS
endothelial
- iNOS
iNOS inducible NOS
- PTP
permeability transition pore
- MPA
mycophenolic acid
- RAPA
Rapamycin
- TACI
transmembrane activator and calcium modulating and cyclophillin ligand interactor
- SLE
systemic lupus erythematosus
- TCR
T cell antigen receptor
- ZAP-70
ζ-associated protein-70
- ΔΨm
mitochondrial transmembrane potential.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects. Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Nagy G, Koncz A, Perl A. T- and B-cell abnormalities in systemic lupus erythematosus. Crit Rev Immunol. 2005;25:123–140. doi: 10.1615/critrevimmunol.v25.i2.30. [DOI] [PubMed] [Google Scholar]
- 3.Skulachev VP. Mitochondrial physiology and pathology; concepts of programmed death of organelles, cells and organisms. Mol.Asp.Med. 1999;20:139–140. doi: 10.1016/s0098-2997(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 4.Constantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J. Biol .Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 5.Perl A, Gergely P, Jr., Puskas F, Banki K. Metabolic switches of T-cell activation and apoptosis. Antiox.Redox Signal. 2002;4:427–443. doi: 10.1089/15230860260196227. [DOI] [PubMed] [Google Scholar]
- 6.Banki K, Hutter E, Gonchoroff N, Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J.Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- 7.Banki K, Hutter E, Colombo E, Gonchoroff NJ, Perl A. Glutathione Levels and Sensitivity to Apoptosis Are Regulated by changes in Transaldolase expression. J.Biol.Chem. 1996;271:32994–33001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- 8.Puskas F, Gergely P, Banki K, Perl A. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 2000;14:1352–1361. doi: 10.1096/fj.14.10.1352. [DOI] [PubMed] [Google Scholar]
- 9.Li P-F, Dietz R, von Harsdorf R. p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by bcl-2. EMBO J. 1999;18:6027–6036. doi: 10.1093/emboj/18.21.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-XL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol.Cell.Biol. 2000;20:5680–5689. doi: 10.1128/mcb.20.15.5680-5689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarlett JL, Sheard PW, Hughes G, Ledgerwood EC, Ku H-H, Murphy MP. Changes in mitochondrial membrane potential during staurosporin-induced apoptosis in Jurkat cells. FEBS Lett. 2000;475:267–272. doi: 10.1016/s0014-5793(00)01681-1. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Alcazar JA, Ault JG, Khodjakov A, Schneider E. Increased mitochondrial cytochrome c levels and mitochondrial hyperpolarization precede camptothecin-induced apoptosis in Jurkat cells. Cell Death Differ. 2000;7:1090–1100. doi: 10.1038/sj.cdd.4400740. [DOI] [PubMed] [Google Scholar]
- 13.Almeida A, Almeida J, Bolanos JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc.Natl.Acad.Sci.USA. 2001;18(Dec):15294–15299. doi: 10.1073/pnas.261560998. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA. HIV-1 Trans Activator of Transcription Protein Elicits Mitochondrial Hyperpolarization and Respiratory Deficit, with Dysregulation of Complex IV and Nicotinamide Adenine Dinucleotide Homeostasis in Cortical Neurons. J..Immunol. 2007;178:869–876. doi: 10.4049/jimmunol.178.2.869. [DOI] [PubMed] [Google Scholar]
- 15.Gergely PJ, Grossman C, Niland B, Puskas F, Neupane H, Allam F, Banki K, Phillips PE, Perl A. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arth..Rheum.. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy G, Koncz A, Perl A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J..Immunol. 2003;171:5188–5197. doi: 10.4049/jimmunol.171.10.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan S, Kiang JG, Fisher CU, Nambiar MP, Nguyen HT, Kyttaris VC, Chowdhury B, Rus V, Tsokos GC. Increased Caspase-3 Expression and Activity Contribute to Reduced CD3{zeta} Expression in Systemic Lupus Erythematosus T Cells. J. Immunol. 2005;175:3417–3423. doi: 10.4049/jimmunol.175.5.3417. [DOI] [PubMed] [Google Scholar]
- 18.Perl A, Gergely P, Jr., Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T cell life, death, and autoimmunity. Trends. Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Ann.Rev.Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 20.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of Cell Death: Exposure to Necrotic Tumor Cells, but Not Primary Tissue Cells or Apoptotic Cells, Induces the Maturation of Immunostimulatory Dendritic Cells. J. Exp.Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisner MD, Amory J, Mullaney B, Tierney L, Jr., Browner WS. Necrotizing lymphadenitis associated with systemic lupus erythematosus. Sem. Arth. Rheum. 1996;26:477–482. doi: 10.1016/s0049-0172(96)80028-x. [DOI] [PubMed] [Google Scholar]
- 22.Ma L, Chan KW, Trendell-Smith NJ, Wu A, Tian L, Lam AC, Chan AK, Lo CK, Chik S, Ko KH, To CK, Kam SK, Li XS, Yang CH, Leung SY, Ng MH, Stott DI, MacPherson GG, Huang FP. Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur. J. Immunol. 2005;35:3364–3375. doi: 10.1002/eji.200535192. [DOI] [PubMed] [Google Scholar]
- 23.Lanzaveccia A, Lezzi G, Viola A. From TcR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 24.Germain RN. The T cell receptor for antigen: signaling and ligand discrimination. J. Biol. Chem. 2001;276:35223–35226. doi: 10.1074/jbc.R100025200. [DOI] [PubMed] [Google Scholar]
- 25.Abraham N, Miceli MC, Parnes JR, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991;350:62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- 26.Samelson LE, Klauser RD. Tyrosine kinases and tyrosine based activation motifs. J. Biol. Chem. 1992;267:24913–2416. [PubMed] [Google Scholar]
- 27.Imboden JB, Weiss A. The T-cell antigen receptor regulates sustained increases in cytoplasmic free Ca2+ through extracellular Ca2+ influx and ongoing intracellular Ca2+ mobilization. Biochem J. 1987;247:695–700. doi: 10.1042/bj2470695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis RS. Calcium signaling mechanism in T lymphocytes. Ann. Rev. Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 29.Premack BA, Gardner P. Signal transduction by T cell receptors: mobilization of Ca and regulation of Ca-dependent effector molecules. Am. J. Physiol. 1992;263:19–40. doi: 10.1152/ajpcell.1992.263.6.C1119. [DOI] [PubMed] [Google Scholar]
- 30.Chance B. The nature of electron transfer and energy coupling reactions. FEBS Letters. 1972;23:3–20. doi: 10.1016/0014-5793(72)80272-2. [DOI] [PubMed] [Google Scholar]
- 31.Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;15:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duchen MR. Topical Review mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic Ca2+ oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 34.Perl A, Qian Y, Chohan KR, Shirley CR, Amidon W, Banerjee S, Middleton FA, Conkrite KL, Barcza M, Gonchoroff N, Suarez SS, Banki K. Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. Proc Natl Acad Sci U S A. 2006;103:14813–14818. doi: 10.1073/pnas.0602678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent Control of the Permeability Properties of the Mitochondrial Outer Membrane and Voltage-dependent Anion-selective Channel (VDAC) J.Biol.Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 36.Bredt DS. Endogenous nitrice oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 37.Song H, Xia SL, Liao C, Li YL, Wang YF, Li TP, Zhao MJ. Genes encoding Pir51, Beclin 1, RbAp48 and aldolase b are up or down-regulated in human primary hepatocellular carcinoma. World J. Gastroenterol. 2004;10:509–513. doi: 10.3748/wjg.v10.i4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiling N, Kroncke R, Ulmer AJ, Gerdes J, Flad HD, Hauschildt S. Nitric oxide synthase: expression of the endothelial, Ca2+/calmodulin-dependent isoform in human B and T lymphocytes. Eur. J. Immunol. 1996;3:511–516. doi: 10.1002/eji.1830260302. [DOI] [PubMed] [Google Scholar]
- 39.Ibiza S, Victor VM, Bosca I, Ortega A, Urzainqui A, O'Connor JE, Sanchez-Madrid F, Esplugues JV, Serrador JM. Endothelial nitric oxide synthase regulates T cell receptor signaling at the immunological synapse. Immunity. 2006;24:753–765. doi: 10.1016/j.immuni.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Beltran B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S. The effect of nitric oxide on cell respiration: a key to undertanding its role in cell survival, or death. Proc. Natl. Acad. Sci. USA. 2000;26:14602–14607. doi: 10.1073/pnas.97.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J. Cell Sci. 2006;119:2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- 42.Nisoli E, Clementi E, Paolucci C. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 43.Nagy G, Barcza M, Gonchoroff N, Philips PE, Perl A. Nitric oxide- dependent mitochondrial biogenesis generates Ca2+ signaling profile of lupus T cells. J. Immunol. 2004;173:3576–3583. doi: 10.4049/jimmunol.173.6.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 45.Bates TE, Loesch A, Burnstock G, Clark JB. Mitochondrial nitric oxide synthase: a ubiquitous regulator of oxidative phosphorylation? Biochem. Biophys. Res. Commun. 1996;218:40–44. doi: 10.1006/bbrc.1996.0008. [DOI] [PubMed] [Google Scholar]
- 46.Valdez LB, Zaobornyj T, Boveris A. Mitochondrial metabolic states and membrane potential modulate mtNOS activity. Biochim. Biophys. Acta. 2006;1757:166–172. doi: 10.1016/j.bbabio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Gao S, Chen J, Brodsky SV, Huang H, Adler S, Lee JH, Dhadwal N, Cohen-Gould L, Gross SS, Goligorsky MS. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J.Biol.Chem. 2004;279:15968–15974. doi: 10.1074/jbc.M308504200. [DOI] [PubMed] [Google Scholar]
- 48.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell. Biol. 2002;3(Mar):214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 49.Cemerski S, Cantagrel A, Van Meerwijk JP, Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J. Biol. Chem. 2002;277:19585–19593. doi: 10.1074/jbc.M111451200. [DOI] [PubMed] [Google Scholar]
- 50.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic. Biol. Med. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 52.Hardwick JS, Sefton BM. Activation of the Lck tyrosine protein kinase by hydrogen peroxide requires the phosphorylation of Tyr-394. Proc. Natl. Acad. Sci. U S A. 1995;92:4527–42531. doi: 10.1073/pnas.92.10.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot (Lond) 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim YM, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ. Res. 1999;19:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- 55.Chung HT, Pae HO, Choi BM, Billiar TR, Kim YM. Nitric oxide as a bioregulator of apoptosis. Biochem. Biophys. Res. Commun. 2001;282:1075–1079. doi: 10.1006/bbrc.2001.4670. [DOI] [PubMed] [Google Scholar]
- 56.Melino G, Catani MV, Corazzari M, Guerrieri P, Bernassola F. Nitric oxide can inhibit apoptosis or switch it into necrosis. Cell Mol. Life. Sci. 2000;57:612–622. doi: 10.1007/PL00000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beltran B, Quintero M, Gracia-Zaragoza E, O'Connor E, Esplugues JV, Moncada S. Inhibition of mitochondrial respiration by endogenous nitric oxide: a critical step in Fas signalling. Proc. Natl. Acad. Sci. USA. 2002;13:8892–8897. doi: 10.1073/pnas.092259799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown CG. Nitric oxide and mitochondrial respiration. Biochem. Biophys. Acta. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 59.Brookes PS, Salinas EP, Darley-Usmar K, Eiserich JP, Freeman BA, Darley-Usmar VM, Anderson PG. Concentration dependent effects of nitric oxide on mitochondrial permeability transition and cytochrome crelease. J. Biol. Chem. 2000;275:20474–20479. doi: 10.1074/jbc.M001077200. [DOI] [PubMed] [Google Scholar]
- 60.Packer MA, Murphy MP. Peroxynitrite causes calcium efflux from mitochondria which is prevented by Cyclosporin A. FEBS Lett. 1994;2−3:237–240. doi: 10.1016/0014-5793(94)00461-7. [DOI] [PubMed] [Google Scholar]
- 61.Weinberg JB, Granger DL, Pisetsky DS. Alterations to glutathione and nicotinamide nucleotides during the mitochondrial permeability transition induced by peroxynitrite. Biochem. Pharmacol. 1996;7:1047–1055. doi: 10.1016/0006-2952(96)99426-5. [DOI] [PubMed] [Google Scholar]
- 62.Packer MA, Murphy MP. Peroxynitrite formed by simultaneous nitric oxide and superoxide generation causes cyclosporin-A-sensitive mitochondrial calcium efflux and depolarisation. Eur. J. Biochem. 1995;1:231–239. doi: 10.1111/j.1432-1033.1995.231_c.x. [DOI] [PubMed] [Google Scholar]
- 63.Caricchio R, Cohen PL. Spontaneous and induced apoptosis in systemic lupus erythematosus: multiple assays fail to reveal consistent abnormalities. Cell. Immunol. 1999;1:54–60. doi: 10.1006/cimm.1999.1576. [DOI] [PubMed] [Google Scholar]
- 64.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenisine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amin AR, Attur M, Vyas P. Expression of nitric oxide synthase in human peripheral blood mononuclear cells and neutrophils. J. Inflamm. 1995;4:190–205. [PubMed] [Google Scholar]
- 66.Paglin S, Lee NY, Nakar C, Fitzgerald M, Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Lampen N, Yahalom J. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005;65:11061–11070. doi: 10.1158/0008-5472.CAN-05-1083. [DOI] [PubMed] [Google Scholar]
- 67.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis. Rheum. 1994;37:289–297. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int. J. Mol. Med. 2004;13:537–543. [PubMed] [Google Scholar]
- 70.Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc..Natl..Acad..Sci..USA. 2002;99:4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]