Abstract
Aims/hypothesis
Type 1 diabetes results from the autoimmune destruction of pancreatic beta cells. Exogenous insulin therapy cannot achieve precise physiological control of blood glucose concentrations, and debilitating complications develop. Lentiviral vectors are promising tools for liver-directed gene therapy. However, to date, transduction rates in vivo remain low in hepatocytes, without the induction of cell cycling. We investigated long-term transgene expression in quiescent hepatocytes in vitro and determined whether the lentiviral delivery of furin-cleavable insulin to the liver could reverse diabetes in rats.
Materials and methods
To improve transduction efficiency in vitro, we optimised hepatocyte isolation and maintenance protocols and, using an improved surgical delivery method, delivered furin-cleavable insulin alone or empty vector to the livers of streptozotocin-induced diabetic rats by means of a lentiviral vector. Rats were monitored for changes in body weight and blood glucose, and intravenous glucose tolerance tests were performed. Expression of insulin was determined by RT-PCR, immunohistochemistry and electron microscopy.
Results
We achieved long-term transgene expression in quiescent hepatocytes in vitro (87 ± 1.2% transduction efficiency), with up to 60 ± 3.2% transduction in vivo. We normalised blood glucose for 500 days—a significantly longer period than previously reported—making this the first successful study using a lentiviral vector. This procedure resulted in the expression of genes encoding several beta cell transcription factors, some pancreatic endocrine transdifferentiation, hepatic insulin storage in granules, and restoration of glucose tolerance. Liver function tests remained normal. Importantly, pancreatic exocrine transdifferentiation did not occur.
Conclusions/interpretation
Our data suggest that this regimen may ultimately be employed for the treatment of type 1 diabetes.
Keywords: Beta cell transcription factors, Gene therapy, Hepatocytes, Insulin storage, Lentiviral vector, Type 1 diabetes, Wistar rats
Introduction
Type 1 diabetes mellitus is caused by autoimmune destruction of pancreatic beta cells [1]. Treatment requires daily injections of insulin to normalise blood glucose levels. Tight glucose control delays, but does not prevent, the onset of complications, which increase morbidity and mortality. Transplantation therapy is limited by the scarcity of donors and chronic immunosuppressive regimens [2]. Physiological glucose control could be achieved by genetically engineering surrogate beta cells that are capable of synthesising, storing and secreting insulin in response to metabolic signals.
One approach to liver-directed gene therapy of diabetes is the transcriptional control of insulin expression without the induction of storage [3, 4], which is nonresponsive to minute-to-minute fluctuations in blood glucose. Another strategy is the delivery of beta cell transcription factors to the liver to induce pancreatic transdifferentiation. Studies have employed transiently expressing adenoviral vectors to deliver pancreatic and duodenal homeobox gene 1 (Pdx1) and neurogenic differentiation gene 1 (Neurod1)/betacellulin gene (Btc) [5–8] to streptozotocin (STZ)-induced diabetic mice and isolated human liver cells, which have resulted in liver-to-pancreas transdifferentiation and reversal of diabetes. However, conversion of significant portions of the liver to ‘true’ pancreatic tissue may lead to an increased risk of autoimmune attack and development of hepatitis, due to the exocrine differentiating capacity of PDX1 [7].
Most studies on the delivery of genes to the liver have employed adenoviral vectors [5–8] due to their ability to efficiently transduce non-dividing cells. However, the therapeutic gene remains episomal, often with consequent short-term gene expression and detrimental host immune responses [9]. Short-term euglycaemia has been achieved following delivery of insulin via an adeno-associated vector, with dose-dependent efficacy [10]. Lentiviral vectors (LV) are capable of stably integrating genes into the chromosomes of dividing and non-dividing cells [9, 11–13]. However, their ability to transduce quiescent hepatocytes in vivo remains low, as efficient transduction generally requires induction of cell cycling [14], which can be achieved by partial hepatectomy [15] or delivery of hepatotoxins [16, 17].
We have developed an improved method for high efficiency transduction of rat hepatocytes in vitro and describe a modified surgical technique for efficient delivery of LV to rat livers resulting in efficient transduction, without the requirement for cell division. We have used this technique to deliver lentivector-encoded furin-cleavable insulin (INS-FUR) [18], which enables cells to cleave proinsulin to mature insulin, in the livers of diabetic rats. This has resulted in permanent reversal of diabetes, insulin storage and restoration of glucose tolerance.
Materials and methods
Vector production INS-FUR cDNA [18] (proins.IfurIIfur.B10D; INS-FUR; gift from Genentech, San Francisco, CA, USA) was cloned into the multi-cloning site of the LV HIV/MSCV (HMD) [19] (a gift from J. Choi, Department of Pathology and Laboratory Medicine, University of Pennsylvania, PA, USA) at the EcoRI site to produce the HMD/INS-FUR construct. This LV vector has an HIV/murine stem cell virus hybrid long terminal repeat as the promoter and an internal ribosomal entry site, allowing bicistronic expression of the genes encoding insulin and enhanced green fluorescent protein (EGFP) simultaneously. The vectors were produced by calcium phosphate precipitation cotransfection of three plasmids (10 μg HMD/INS-FUR or 10 μg pHR’CMVGFP [20] expressing EGFP alone, plus 20 μg pCMVΔR8.2 and 10 μg of the heterologous VSV-G envelope construct pCVSV-G; all other vector constructs were gifts from I. Verma, Salk Institute for Biological Sciences, CA, USA) into 293T cells [9]. Conditioned medium was collected 48 h after transfection, filtered and pelleted (50,000 g). Virus titre was determined by transducing 293T cells (5 × 105) with serially diluted vector stocks and quantifying numbers of EGFP-positive cells by flow cytometry as described [21]. Viral replication-competency was also assessed.
Transduction of hepatocytes in vitro Maintenance and experimental manipulation of male Wistar rats (250–300 g; Gore Hill Research Laboratories, Sydney, NSW, Australia) were performed in accordance with the NIH principles of laboratory care and regulations of the Australian Research Council and were approved by the University of Technology Sydney. Hepatocytes were isolated using a two-step EDTA/collagenase digestion. The portal vein (PV) and the abdominal aorta were cannulated and the liver was flushed in situ with warmed (40°C) Hanks’ buffered salt solution without Ca2+ and Mg2+ (Sigma-Aldrich, Castle Hill, NSW, Australia), plus 1 mmol/l EDTA for 5–7 min. This was followed by perfusion with 0.05% collagenase (C5138; Sigma-Aldrich) in Hanks’ buffered salt solution with Ca2+ and Mg2+ (Sigma-Aldrich) for 3–5 min. The hepatocytes were collected, filtered through sterile 250 and 100 μm mesh and washed three times in DMEM plus 10% FCS. Preparations containing greater than 85% viable cells were plated onto Matrigel (gift from C. Liddle, Westmead Hospital, Sydney, NSW, Australia) and cultured in DMEM supplemented with NaHCO3 (44 mmol/l), insulin (100 mU/l), glutamic acid (2.5 mmol/l), proline (1 mmol/l), ascorbic acid (56 mg/l), penicillin (100 mg/ml), nicotinamide (10 mmol/l), epidermal growth factor (20 ng/ml), selenium salts (0.1 μg/ml) and hydrocortisone (1 nmol/l). After 5 days, 1.5% DMSO together with CuSO4 (2.5 μg/l), FeSO4.7H2O (834 μg/l) and ZnSO4 (1.47 mg/l) was also added to the medium. Although Matrigel inhibits cell division, hepatocytes were also irradiated (caesium-137 source; total doses of 0, 10 or 20 Gy) 24 h after seeding and DNA synthesis was assessed by [3H]thymidine incorporation [22]. The positive control was hepatocytes plated on rat-tail collagen 1 (Collaborative Biomedical Products, Bedford, MA, USA).At 24 h after cell attachment, hepatocytes (5×105 per well) were transduced with pHR’CMVGFP LV (2.5 × 107 transduction units [TDU]) for 16 h in the presence of 8 μg/ml polybrene. Transduction efficiency was assessed at 2, 7, 14, 21 and 28 days by fluorescence microscopy and flow cytometry. EGFP-expressing cells were also stained for the liver-specific marker glucose-6-phosphatase.
Transduction of hepatocytes in vivo Rats were divided into four groups of 16 animals. For Group 1, the right adrenal vein was ligated and successively the infrahepatic vena cava (IVC), hepatic artery, PV and suprahepatic vena cava were clamped before pHR’CMVGFP or HMD LV (5 × 108 TDU) was infused through the PV. During this procedure, blood supply to the liver was stopped for 5 min and then recommenced for 2 min. This procedure of intervallic infusion in full flow occlusion (FFO) was repeated three times. Animals in Group 2 were treated similarly; except 0.9% saline was infused. A 70% partial hepatectomy was performed on animals in Group 3 by resecting the left lateral and median liver lobes. The right adrenal vein was ligated and LV was infused 24 h later by the procedure detailed for Group 1. A fourth group of animals was treated the same as Group 3, except 0.9% saline was infused. Livers were removed at 1, 2, 4 and 8 weeks. Transduction efficiency was assessed by flow cytometric quantification of EGFP-positive cells.To assess cell proliferation, 5-bromo-2′-deoxyuridine (100 mg/kg; Sigma) was injected intraperitoneally at 18, 28 and 48 h after surgery (partial hepatectomy or sham-operated) and into untreated animals (three animals per time-point). After 2 h, livers were excised and processed by conventional histological techniques. Incorporation of 5-bromo-2′-deoxyuridine was determined by immunohistochemistry.Diabetes was induced by an intraperitoneal injection of STZ (75 mg/kg body weight; Sapphire Science, Crows Nest, NSW, Australia). Animals received HMD/INS-FUR or empty HMD vector administered by FFO without partial hepatectomy. After treatment, animals were monitored for body weight and blood glucose. Animals were killed at 60 or 500 days and body tissues were sampled. To determine the proportion of EGFP-expressing cells that were hepatocytes, liver cells were isolated and stained with anti-cytokeratin 18 (CK18) antibody (Chemicon, Temecula, CA, USA) and R-phycoerythrin-conjugated secondary antibody (Chemicon). Numbers of insulin-positive cells were determined using an anti-insulin antibody (Sigma-Aldrich) and aminomethylcoumarin-conjugated secondary antibody followed by flow cytometric analysis.
Microscopic analyses Livers and pancreases were collected 60 and 500 days after vector infusion. Islet hormones were identified by peroxidase immunohistochemistry using diaminobenzidine as substrate. Primary antibodies against insulin (BioGenex, San Ramon, CA, USA), glucagon (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and somatostatin (Santa Cruz Biotechnology) were used. All cells in ten random fields were scored. Data were expressed as the number of positive cells per mm2 of tissue.For electron microscopy, tissue was fixed and processed using uranyl acetate block-staining and lead citrate counter-staining of ultra-thin sections (80 nm). For insulin immunoelectronmicroscopy, a post-embedding immunogold procedure was used. The beta cell line Nit-1 was the positive control. Tissues and cells were embedded in LR white and labelling procedures were performed as previously described [23].
Functional studies Intravenous glucose tolerance tests were performed on fasted (6–8 h) animals. After infusion of glucose (0.5 g/kg body weight), blood samples were collected from the tail vein. Insulin content of pancreas and liver was determined after acid/ethanol extraction [23].Insulin and proinsulin in acid/ethanol extractions were separated on a Sephadex G50 superfine column (Sigma-Aldrich) [24]. Insulin in serum samples and column fractions was measured using an RIA for human insulin (Linco Research, St Charles, MO, USA), which has less than 1 and 6% cross-reactivity with rodent insulin and human proinsulin, respectively. Commercial RIAs (Linco Research) were used to measure human and rat C-peptide [25].To assess liver function, aspartate transaminase (AST) and alanine aminotransferase (ALT) were measured daily over the first 10 days and then monthly using commercial kits (Roche Diagnostics, Castle Hill, NSW, Australia).
PCR analyses For RT-PCR, liver, pancreas, spleen, kidney and lung were collected at 60 and 500 days after vector infusion and snap-frozen in liquid nitrogen. Total RNA was extracted using Trizol (Invitrogen Australia, Mt Waverly, VIC, Australia). Samples were treated with DNase I (Invitrogen). Real-time quantitative PCR was conducted using SYBR green I and a sequence detection system (ABI 7500 HT; Applied Biosystems, Scoresby, VIC, Australia). Optimal primer and cDNA template concentrations were determined by titration. Amplification efficiencies for each primer set were determined to be similar. Data were represented as differences between threshold cycle values (ΔCT) for the transcripts of interest and the internal standard, β-actin. The expression of the genes encoding human insulin and EGFP was examined in normal and HMD/INS-FUR-transduced liver cells using semi-quantitative RT-PCR. Oligonucleotide sequences are available on request.
Qualitative western blot analyses Nuclear and cytoplasmic extracts of liver and pancreas were prepared using a nuclear extraction Kit (Chemicon). Samples (1 μg protein) were separated by SDS-PAGE and transferred to nitrocellulose. Primary antibodies against PDX1 and NEUROD1 (Santa Cruz) were used.
Statistical analyses Differences between groups were determined by Student’s paired t test or, if there were more than two groups, by one-way analysis of variance after log transformation of data. Data are expressed as means ± standard errors (SEM).
Results
Efficient transduction of hepatocytes Isolation of hepatocytes by dual perfusion of the PV and abdominal aorta resulted in increased cell viability as compared with perfusion of the PV alone (85.7 ± 6.2% vs 67.5 ± 7.8%, respectively, p = 0.03). The cells were morphologically normal, as assessed by light and electron microscopy, with maintenance of gap junctions between cells and limited vacuolation. Hepatocytes grown on Matrigel in the optimised medium, with or without irradiation, did not undergo cell division, as assessed by [3H]thymidine incorporation. Cells grown on collagen, which enhances cell growth, reached a maximum growth rate at day 2 and this plateaued between days 5 and 6 (data not shown). Expression of EGFP was seen 48 h post-transduction and persisted for over 1 month (Fig. 1a). Up to 87 ± 1.2% of the irradiated cells expressed EGFP (Fig. 1b). Most EGFP-positive cells (80.2 ± 10.7%) were also positive for glucose-6-phosphatase, which suggested a hepatocyte lineage (data not shown). Growth of hepatocytes on collagen did not increase transduction efficiency. Delivery of HMD/INS-FUR to hepatocyte cultures resulted in low constitutive insulin expression (0.2 pmol per 3 × 105 cells) and no insulin storage; therefore transplantation of hepatocytes transduced in vitro was not performed. We delivered pHR’CMVGFP to the livers of diabetic rats by FFO and compared the transduction efficiency in non-hepatectomised and partially-hepatectomised animals. The total wet weights of liver 48 h after partial hepatectomy were similar to those of non-hepatectomised rats (14.3 ± 0.4 and 14.7 ± 0.8 g, respectively). We also determined whether FFO stimulated cell division. In control rats, 0.1 ± 0.06% of hepatocytes were proliferating at 18 h and this rate did not significantly alter at any time point. At 18 h in animals that had undergone FFO, 0.3 ± 0.2% of hepatocytes were proliferating, compared with 0.4 ± 0.3% at 28 h and 0.1 ± 0.04% at 48 h. These values were not significantly different to those recorded for control animals. By comparison, in animals that had undergone a partial hepatectomy, 12.8 ± 2.4% of hepatocytes were proliferating at 18 h. This value increased to 21.4 ± 4.3% at 28 h and then decreased to 7.4 ± 1.2% at 48 h.To determine transduction efficiency, liver cells were isolated after transduction with pHR’CMVGFP and EGFP-positive cells were assessed by flow cytometry. The maximal number of EGFP-expressing cells was detected 2 weeks after transduction (57.0 ± 2%) and was not significantly different after 2 months (58.2 ± 3.5%). In animals that were partially hepatectomised prior to transduction, there was no significant difference in numbers of EGFP-expressing hepatocytes (59.2 ± 4%) after 2 months compared with non-hepatectomised animals. Use of the HMD vector also resulted in high levels of transduction (Fig. 1c). Cells expressing EGFP/INS-FUR were almost exclusively hepatocytes, as verified by CK18 and insulin labelling (Fig. 1d,e).
Fig. 1.
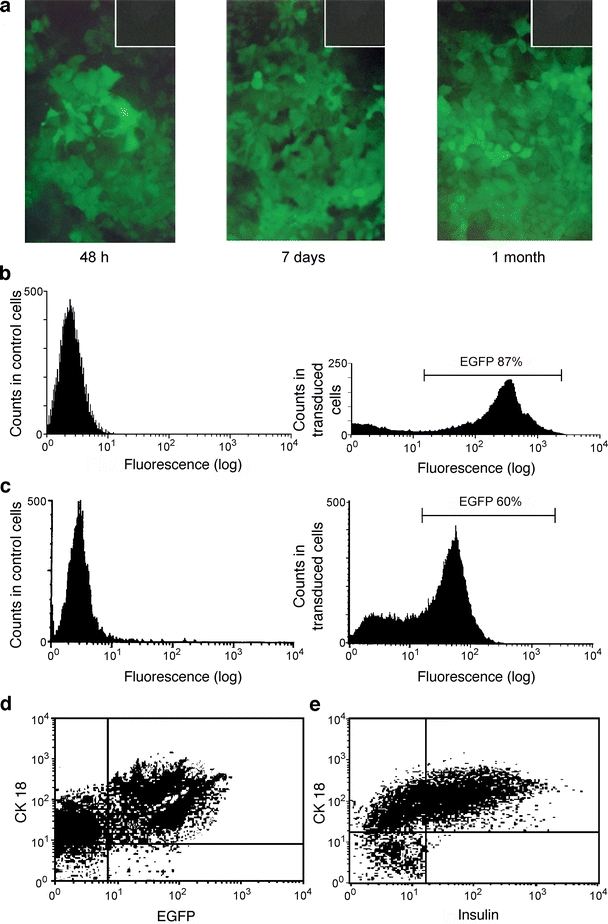
EGFP expression in hepatocytes in vitro and in vivo. a EGFP expression in primary hepatocytes in vitro at indicated times post transduction with pHR’CMVGFP. Original magnification: 400×. The insets show untransduced hepatocytes. b Flow cytometric analysis of irradiated (10 Gy), non-dividing primary hepatocytes 14 days post-transduction with pHR’CMVGFP. Negative control cells show background fluorescence only. EGFP-positive cells constituted 87 ± 1.2% of all cells following transduction. Graphs representative of three independent experiments. c Flow cytometric analysis of liver cells isolated from a normal control rat and a rat transduced with the HMD LV vector 1 month previously. EGFP-positive cells constituted 60 ± 3.2% of all liver cells. The results are representative of three experiments. d Representative flow cytometry dot-plot showing the proportion of EGFP-expressing cells that were hepatocytes (CK-18-positive: 58 ± 3.1%) from an animal that had been transduced with the HMD/INS-FUR LV 2 months previously and e the proportion of insulin-positive (57 ± 2.8%) hepatocytes. Graphs are representative of three experiments
Transduction with HMD/INS-FUR reversed STZ-diabetes Blood glucose levels of STZ-diabetic rats decreased to subnormal levels immediately after transduction with HMD/INS-FUR vector (Fig. 2a). By day 5, blood glucose levels of insulin-transduced animals were not different to non-diabetic controls and reversal of diabetes was maintained for 500 days (experimental endpoint). STZ-diabetic rats treated with HMD alone remained hyperglycaemic until day 60, when they were killed. The body weights of rats treated with HMD/INS-FUR were not significantly different to those of non-diabetic controls until day 125, lower between days 135 and 275, becoming not significantly different again after day 300 (Fig. 2b). The body weights of rats treated with the empty vector alone continued to fall from day 50 to 60. Throughout the experimental period, there was no elevation of liver enzymes in animals treated with HMD/INS-FUR (AST: 38.4 ± 6, ALT: 26.9 ± 5 U/l) compared with control animals (AST: 44.2 ± 16, ALT: 19.1 ± 7).
Fig. 2.
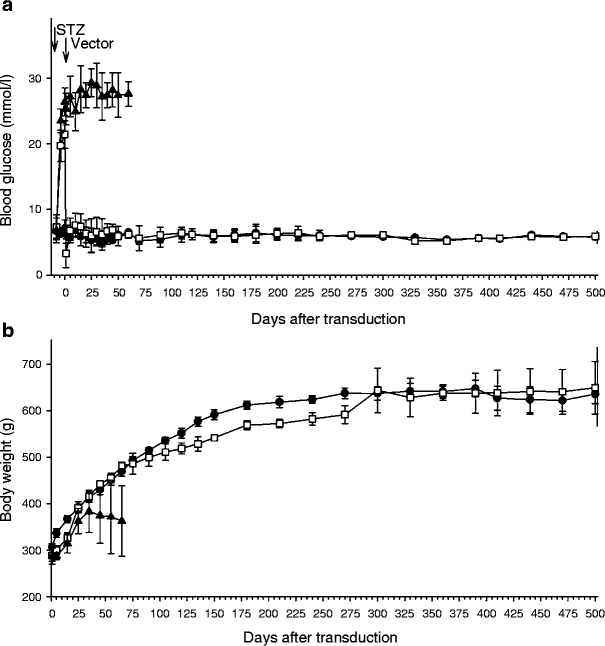
Stable expression of INS-FUR ameliorated STZ-induced hyperglycaemia. Blood glucose concentrations (a) and body weights (b) of non-diabetic control rats (black circles) and STZ-diabetic rats treated with HMD/INS-FUR (white squares) or HMD alone (black triangles). Values are means±SEM (n = 5)
HMD/INS-FUR transduction stimulated expression of pancreatic hormones We examined pancreatic hormone-producing cells in the livers of rats transduced with LV after 2 months. Figure 3a shows extensive staining for insulin in normal pancreatic islets. By comparison, pancreatic beta cells were rarely observed in STZ-diabetic animals (1.5 ± 1.4 cells) (Fig. 3b). Normal liver was negative for insulin (Fig. 3c), glucagon and somatostatin (not shown). No pancreatic hormones were detected in livers of animals treated with empty vector. Following transduction with HMD/INS-FUR, significant numbers of hepatocytes were converted into insulin-positive cells (Fig. 3d). There was also evidence of some pancreatic transdifferentiation, as glucagon- and somatostatin-positive cells were detected (Fig. 3e,f). Cells positive for pancreatic hormones were distributed throughout the livers of animals transduced with HMD/INS-FUR and not restricted to regions adjacent to the PV. Electron microscopy revealed cytoplasmic granules (270–330 nm in diameter) in liver tissue from animals treated with HMD/INS-FUR (Fig. 3g). Immunoelectron microscopy of insulin-producing cells in the liver of STZ-diabetic rats transduced with HMD/INS-FUR was comparable to those observed in Nit-1 beta cells (Fig. 3h,i). Inflammatory cell infiltration and necrosis within the livers of transduced animals were not observed. Insulin-containing granules were absent in rats treated with empty vector and in untreated animals.
Fig. 3.
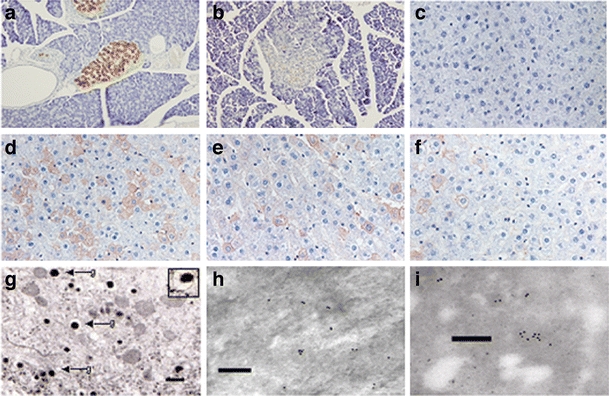
Expression of pancreatic hormones following reversal of STZ-induced hyperglycaemia. Photomicrographs of anti-insulin staining of (a) normal pancreas, (b) STZ-diabetic HMD/INS-FUR vector-treated pancreas, (c) normal liver and (d) STZ-diabetic HMD/INS-FUR vector-treated liver at 2 months. Photomicrographs of anti-glucagon (e) and anti-somatostatin (f) staining in STZ-diabetic HMD/INS-FUR vector-treated liver at 2 months. Positive cells appear brown. Original magnification: 200×. In a total of 1,200 ± 61 cells the number of insulin-positive cells (585.6 ± 73.8 cells/mm2) was fivefold higher than the number of glucagon-positive cells (109.9 ± 37.1 cells/mm2) and 11-fold higher than the number of somatostatin-positive cells (52.2 ± 8.1 cells/mm2). The number of EGFP-positive cells was 854 ± 80.9 (data not shown). Therefore, some EGFP-positive cells did not store pancreatic hormones. No pancreatic hormones were detected in liver from animals treated with empty vector or from control animals. g Transmission electron micrograph showing secretory vesicles with dense granules surrounded by a pale halo (bar: 500 nm). Inset, vesicle 320 nm in diameter. h Immuno-electron micrographs showing localisation of insulin in liver 2 months after the transduction of an STZ-diabetic rat with HMD/INS-FUR and (i) insulin-secreting granules in Nit-1 beta cells (positive control); bar: 400 nm
HMD/INS-FUR transduction induced hepatic insulin production Insulin storage was 4,165 ± 180 pmol per liver in HMD/INS-FUR-treated animals, compared with 10,418 ± 210 pmol in normal rat pancreas (n = 3). STZ-treated rat pancreas only contained 255±110 pmol insulin. Separation of insulin from proinsulin in liver extracts from animals treated with HMD/INS-FUR yielded two distinct peaks. The first represented proinsulin and partially processed material and the second larger peak corresponded to fully processed insulin (Fig. 4).
Fig. 4.
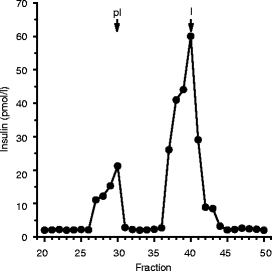
Stable expression of INS-FUR resulted in production of mature human insulin. Extracts of rat livers were separated by gel permeation chromatography and assayed for insulin. Arrows indicate mobility of mature human insulin (I) (5,800 Da) and proinsulin (pI) (9,400 Da). Results are representative of three independent experiments
HMD/INS-FUR transduced animals displayed regulated insulin secretion There was no significant difference between the ability of STZ-diabetic animals transduced with the HMD/INS-FUR vector and untreated animals to normalise blood glucose levels after delivery of a bolus of intravenous glucose (Fig. 5a). Levels of human C-peptide and human insulin in serum samples from HMD/INS-FUR-treated animals (Fig. 5b,c) were comparable to levels of rat C-peptide and insulin recorded for non-diabetic animals. Negligible levels of rat C-peptide were detected in serum from HMD/INS-FUR-treated STZ-diabetic animals (Fig. 5c).
Fig. 5.
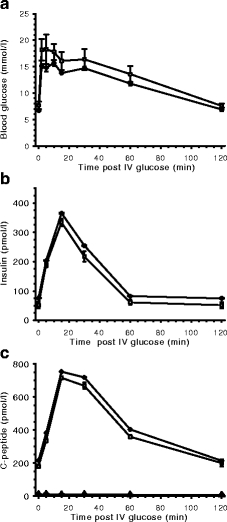
Plasma glucose (a), insulin (b) and C-peptide (c) levels after IVGTT. IVGTTs remained normal for animals maintained for 500 days (data not shown). White squares, HMD/INS-FUR-treated animals (60 days) expressing human insulin and human C-peptide; black diamonds, normal control animals expressing rat insulin and rat C-peptide; black triangles, rat C-peptide in HMD/INS-FUR-treated animals. Values are means±SEM (n = 5)
HMD/INS-FUR transduction stimulated expression of beta cell transcription factors EGFP expression was only detected in liver tissue of pHR’CMVGFP- (Fig. 6a) and HMD-treated (data not shown) rats. Similarly, INS-FUR expression was restricted to the livers of HMD/INS-FUR-treated rats (Fig. 6a). Insulin was not detected in livers from diabetic control animals and diabetic animals injected with HMD alone (data not shown). Mean ratios of insulin : β-actin (1.000 ± 0.069) and EGFP : β-actin (1.088 ± 0.062) in HMD/INS-FUR liver, determined by semi-quantitative PCR, were not significantly different. Transduction of STZ-diabetic rats with the HMD/INS-FUR vector induced sustained expression of several pancreatic transcription factors (Fig. 6b). Normal liver and untreated diabetic liver (not shown) were negative for all pancreatic transcription factors, while normal pancreas was positive. Unexpectedly, both Pdx1 and Neurod1 expression were detected by RT-PCR in empty vector-transduced livers; however, protein production was not detected (Fig. 6c). HMD/INS-FUR-transduced livers also expressed the genes for neurogenin 3 (Neurog3) and Nkx2-2 (Nkx2-2). Glucagon and somatostatin were present in liver transduced with HMD/INS-FUR (confirmed by immunohistochemistry; Fig. 3e,f). Pancreatic polypeptide was not expressed. The transduction of diabetic animals with HMD/INS-FUR did not result in complete pancreatic transdifferentation of liver tissue since expression of the rat insulin genes (Ins1, Ins2) and the genes encoding the insulin prohormone convertase 2 (Pc2, also known as Pcsk2) and the exocrine marker p48 (Ptf1a, also known as P48) were not detected (Fig. 6b). Quantitative real time PCR analysis of the expression levels of beta cell transcription factors Pdx1 (4.3 ± 0.9), Neurod1 (5.7 ± 1.1) and Neurog3 (7.4 ± 1.8) in HMD/INS-FUR-treated livers at 500 days indicated that they were lower (p < 0.001) than levels expressed in normal pancreatic tissue (Pdx1: 12.5 ± 2.3, NeuroD1: 16.8 ± 1.4, Neurog3: 18.5 ± 2.2).
Fig. 6.
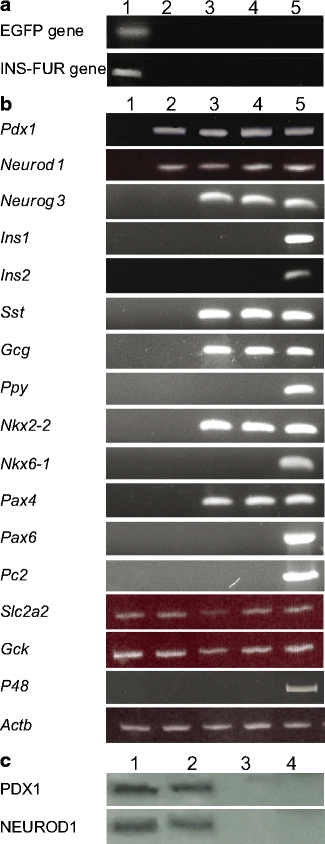
Beta cell transcription factors and pancreatic hormones expressed in transduced rat livers. a RT-PCR analysis of transcription of the genes encoding EGFP or INS-FUR in animals transduced with pHR’CMVGFP or HMD/INS-FUR LV, respectively: liver (lane 1), pancreas (lane 2), spleen (lane 3), kidney (lane 4) and brain (lane 5) at 60 days. Results for HMD/INS-FUR transduced animals at 500 days were identical (not shown). b RT-PCR analysis of transcription of the genes for: (1) beta cell transcription factors (Pdx1, Neurod1, Neurog3, Nkx2-2, Nkx6-1, Pax4, Pax6); (2) the pancreatic endocrine hormones somatostatin (Sst), glucagon (Gcg) and pancreatic polypeptide (Ppy); (3) GLUT2 (Slc2a2) and glucokinase (Gck); (4) rat insulin 1 and 2 (Ins1, Ins2); (5) insulin proconvertase PC2 (Pc2); (6) the exocrine marker p48 (P48); and (7) β-actin (Actb) in normal liver (lane 1), liver transduced with HMD alone at 60 days (lane 2), liver transduced with HMD/INS-FUR LV at 60 days (lane 3) and 500 days (lane 4), and normal pancreas (lane 5). c Western blot analysis for PDX1 and NEUROD1 in normal pancreas (lane 1), liver transduced with HMD/INS-FUR LV at 60 days (lane 2), liver transduced with HMD alone at 60 days (lane 3) and normal liver (lane 4). PDX1 and NEUROD1 generated bands at 43 and 50 kDa, respectively
Discussion
This is the first report of permanent amelioration of STZ-induced hyperglycaemia using LV delivery of INS-FUR. Transduced animals processed, stored and secreted insulin in a glucose-regulated manner. Remission of diabetes, due to the secretion of insulin from residual beta cells, was discounted as insulin-positive pancreatic beta cells were rarely observed and levels of rat C-peptide were negligible. This is also the first report showing that transduction of INS-FUR alone can induce expression of several beta cell transcription factors resulting in hepatocyte differentiation along a pancreatic lineage.
All previous reports of liver–pancreas transdifferentiation have been described in cells that endogenously express beta cell transcription factors [23, 26] or have been engineered to express these factors, leading to varying degrees of transdifferentiation [5–8, 27, 28]. Following immediate reduction in blood glucose due to transgene expression, induction of long-term euglycaemia in the present study was probably related to partial hepatic-to-pancreas transdifferentiation triggered by the lentiviral intrahepatic delivery method of INS-FUR or from expression of stably integrated transgene in the host genome. The HMD/INS-FUR transgene alone, which constitutively expresses insulin, could not mimic the glucose-sensitive kinetics demonstrated by IVGTT, nor could it have solely induced insulin storage. Evidently, the hepatic delivery of the LV was a key factor, for expression of HMD alone triggered expression of Pdx1 and Neurod1, if only at the mRNA level. It is possible that the use of B10-insulin may have been another factor. This insulin is superior to normal insulin in biological activity [29] and has some mitogenic properties [30–32]. However, there was no evidence of excessive mitogenic activity or tumour development, while liver function tests remained normal throughout, with no lymphocytic infiltrates. Parallels can be drawn to the partial pancreatic transdifferentiation observed in Huh7ins cells, which endogenously produce NEUROD1 [23] and PDX1 (C. Tao, University of Technology Sydney; unpublished results). This cell line was transfected with normal human insulin under the control of a constitutive promoter. After this, secretory granules developed, which stored di-arginyl insulin and secreted it in a glucose-responsive manner, correcting diabetes. However, in Huh7ins cells the expression of fully processed native insulin was absent. Similarly, in the present study, pancreatic transdifferentiation was incomplete, as genes encoding transcription factors such as NKX6-1 and PAX6, which occur late in the hierarchy; were not expressed, nor were the rodent insulin genes or the genes for PC2 pancreatic polypeptide or exocrine markers. Therefore, the problems encountered with non-selective pancreatic transdifferentiation, produced after expression of PDX1 alone [5–7, 33, 34], were not encountered.
How the hepatic delivery of INS-FUR induced the expression of Pdx1 and other beta cell transcription factors requires further investigation. Liver and pancreas derive from common progenitor cells. Transdifferentiation between liver and pancreas is controlled by only a few transcription factors [35, 36]. Pdx1 is one such master switch gene. In a recent study, liver cells producing pancreatic markers (insulin and PDX1) were induced and localised to areas adjacent to or within injured tissue areas [37]. Lentiviral transduction may represent a cellular insult, making progenitor cells permissive to a pancreatic developmental shift.
The mechanisms that prevented both complete pancreatic transdifferentiation and expression of endogenous rat insulin are yet to be elucidated. Studies on the development of the rat pancreas indicate that chronologically glucagon and somatostatin appear prior to insulin [38]. The production of these pancreatic hormones may have prevented hypoglycaemia by providing counter-regulatory mechanisms. Complete transdifferentiation into true beta cells may require additional external factors, such as prolonged hyperglycaemia [39, 40]. If this is true, then the rapid reversion to euglycaemia following delivery of the insulin gene in our study may have prohibited complete liver-to-pancreas transdifferentiation. Additionally, the expression of human insulin may have exerted a negative feedback mechanism to inhibit endogenous insulin expression.
Unlike other studies that have engineered beta cell neogenesis from liver cells [5–7], the insulin-containing cells were not isolated to the area surrounding the portal circulation. Insulin storage was 40% of the insulin content of a rat pancreas, satisfying clinical requirements for insulin independence [41]. Corroborating previous studies using INS-FUR, granules predominately stored fully processed insulin [18, 42, 43]. EGFP and insulin were produced at equivalent levels in liver tissue transduced with HMD/INS-FUR. The majority of cells that expressed EGFP and insulin were also CK18-positive, indicative of a hepatocyte lineage for transduced cells.
Despite the lack of the central polypurine tract, LV pHR’CMVGFP efficiently transduced non-dividing hepatocytes in vitro, in the absence of cell division. We obtained an improvement in transduction efficiency of up to 87% in vitro, compared with 68% obtained by Zahler et al. [44]. Increased transduction efficiency may be attributable to several factors, including: (1) the isolation of hepatocytes by perfusion of both the PV and abdominal aorta as opposed to the PV alone, the most widely employed procedure [45]; (2) the optimisation of growth medium; (3) the use of LV at high multiplicity of infection (50:1); and/or (4) the use of the R8.2 packaging plasmid, which encodes accessory HIV proteins. The use of the attenuated plasmid R8.91, used by Zahler et al., resulted in a 5% reduction in transduction efficiency (B. Ren, unpublished observations). Similarly, delivery of INS-FUR in the HMD hybrid vector, which lacks the central polypurine tract [19], to the portal circulation of STZ-diabetic rats by FFO resulted in up to 60% transduction of liver cells, without inducing cell division and with transgene expression being confined to the liver. The FFO technique, which employs repeated vector infusion, is probably responsible for the increased transduction efficiency and is more clinically applicable than the widely used perfusion method, where the IVC is cannulated, thereby increasing the risk of IVC narrowing and pelvic limb thrombosis [46, 47].
This novel approach offers potential as a beta cell replacement strategy with diabetic patients serving as their own donors. Current studies in our laboratory using nonobese diabetic mice, indicate that this procedure was able to reverse autoimmune diabetes for 5 months with no evidence of autoimmune destruction of insulin-secreting hepatocytes (B. Ren, unpublished observations), giving hope that this procedure will be useful for reversal of autoimmune diabetes. There are distinct advantages to the use of LV for transduction of hepatocytes. LV stably integrates into the genome of non-dividing cells, including hepatocytes, and could provide long-lasting expression of therapeutic genes [48, 49]. Whilst LV integration was not specifically examined in this study, the longevity of INS-FUR and EGFP expression provides some evidence for this. Further, human hepatocytes may be more susceptible to LV transduction [50] than rodent cells. LV are probably superior to adenoviral vectors where problems of short-term expression of genes and immunological responses have been reported [9]. Adeno-associated vectors are extremely efficient at transducing mouse livers; however, a recent study using adeno-associated viral 8-mediated delivery of Pdx1 did not result in correction of STZ-diabetes in mice [28]. The unique results reported in this study point to possible benefits of gene therapy using LV.
Acknowledgements
We thank B. Booth, M. Camacho, D. Ernst, Y. Lei, K. Ma, B. Syzmanska (University of Technology Sydney), H. Guo, H. Jullie, B. Zhang (Prince Charles Hospital, Brisbane, QLD, Australia), A. Bishop, C. Wang (Centenary Institute, Royal Prince Alfred Hospital, Sydney, NSW, Australia), B. Tuch (Prince of Wales Hospital, Sydney, NSW, Australia), M. Smith (University of New South Wales, Sydney, NSW, Australia) and R. Limburg. Financial support: National Health and Medical Research Council of Australia (grant no. 9936162), Juvenile Diabetes Research Foundation (grant no. 1-1998-65), Roche Organ Transplant Research Foundation (grant no. 8832450), Rebecca L. Cooper Medical Research Foundation, and University of Technology Sydney.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Abbreviations
- AST
aspartate transaminase
- ALT
alanine aminotransferase
- CK18
cytokeratin 18
- EGFP
enhanced green fluorescent protein
- FFO
intervallic infusion in full flow occlusion
- HMD
lentiviral vector HIV/ MSCV
- INS-FUR
furin-cleavable insulin
- IVC
infrahepatic vena cava
- LV
lentiviral vector
- NEUROD1
neurogenic differentiation protein 1
- PC2
prohormone convertase 2
- PDX1
pancreatic and duodenal homeobox protein 1
- PV
portal vein
- STZ
streptozotocin
- TDU
transduction units
References
- 1.Eisenbarth GS (1986) Type I diabetes mellitus: a chronic autoimmune disease. N Engl J Med 4:1360–1368 [DOI] [PubMed]
- 2.Paty BW, Ryan EA, Shapiro AM, Lakey JR, Robertson RP (2002) Intrahepatic islet transplantation in type I diabetic patients does not restore hyperglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes 51:3428–3434 [DOI] [PubMed]
- 3.Thule JM, Liu J, Phillips LS (2000) Glucose regulated production of human insulin in rat hepatocytes. Gene Ther 7:205–214 [DOI] [PubMed]
- 4.Yoon JW, Jun HS (2002) Recent advances in insulin gene therapy for type I diabetes. Trends Mol Med 8:62–68 [DOI] [PubMed]
- 5.Ferber S, Halkin A, Cohen H et al (2000) Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycaemia. Nature Med 6:568–572 [DOI] [PubMed]
- 6.Ber I, Shternhall K, Perl SI et al (2000) Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem 278:31950–31957 [DOI] [PubMed]
- 7.Kojima H, Fujimiya M, Matsumara K et al (2003) NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nature Med 9:596–603 [DOI] [PubMed]
- 8.Sapir T, Shternhall K, Meivar-Levy I et al (2005) Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A 102:7964–7969 [DOI] [PMC free article] [PubMed]
- 9.Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH (1997) Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol 71:6449–6641 [DOI] [PMC free article] [PubMed]
- 10.Park YI, Woo S, Lee GT et al (2005) Safety and efficacy of adeno-associated viral vector-mediated insulin gene transfer via portal vein to the livers of streptozotocin-induced diabetic Sprague–Dawley rats. J Gene Med 7:621–629 [DOI] [PubMed]
- 11.Kafri T, Blomer U, Petersen DA, Gage FH, Verma IM (1997) Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nature Genet 17:314–317 [DOI] [PubMed]
- 12.Verma IM, Somia N (1997) Gene therapy—promises, problems and prospects. Nature 389:239–242 [DOI] [PubMed]
- 13.Follenzi A, Sabatino G, Lombardo A, Boccaccio C, Naldini L (2002) Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Human Gene Ther 13:234–260 [DOI] [PubMed]
- 14.Park F, Ohashi K, Chiu W, Naldini L, Kay MA (2002) Efficient lentiviral transduction of liver requires cell cycling in vivo. Nature Genet 24:49–52 [DOI] [PubMed]
- 15.Higgins G (2001) Experimental pathology of the liver. Arch Pathol 12:186–202
- 16.Malik R, Mellor N, Seldon C, Hodgson H (2003) Triiodothyronine enhances the regenerative capacity of the liver following partial hepatectomy. Hepatology 37:79–86 [DOI] [PubMed]
- 17.Guidotti JE, Mallet VO, Mitchell C et al (2001) Fas/CD95 pathway induces mouse liver regeneration and allows for highly efficient retrovirus-mediated gene transfer. Hepatology 33:10–15 [DOI] [PubMed]
- 18.Groskreutz DJ, Sliwkowski MX, Gorman CM (1994) Genetically engineered proinsulin constitutively processed and secreted as mature active insulin. J Biol Chem 269:6241–6245 [PubMed]
- 19.Choi JK, Hoang N, Vilardi AM, Conrad P, Emerson SG, Gewirtz AM (2001) Hybrid HIV/MSCV LTR enhances transgene expression of lentiviral vectors in human CD34+ hematopoietic cells. Stem Cells 19:236–246 [DOI] [PubMed]
- 20.Miyoshi H, Takahashi M, Gage FH, Verma IM (1997) Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci U S A 94:10319–10323 [DOI] [PMC free article] [PubMed]
- 21.Logan AC, Nightingale SJ, Haas DL, Cho GJ, Pepper KA, Kohn DB (2004) Factors influencing the titer and infectivity of lentiviral vectors. Hum Gene Ther 15:976–988 [DOI] [PubMed]
- 22.Ott M, Stockert, RJ, Ma Q, Gagandeep S, Gupta S (1998) Simultaneous up-regulation of viral receptor expression and DNA synthesis is required for increasing efficiency of retroviral hepatic gene transfer. J Biol Chem 273:11954–11961 [DOI] [PubMed]
- 23.Tuch BE, Szymanska B, Yao M et al (2003) Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene Ther 10:490–503 [DOI] [PubMed]
- 24.Short DK, Okada S, Yamauchi K, Pessin JE (1998) Adenovirus-mediated transfer of a modified human proinsulin gene reverses hyperglycaemia in diabetic mice. Am J Physiol 275:E748–E756 [DOI] [PubMed]
- 25.Akpan JO, Weide LG, Gingerich CW (1993) A specific and sensitive radioimmunoassay for rat C-peptide. Int J Pancreatol 13:87–95 [DOI] [PubMed]
- 26.Simpson AM, Marshall GM, Tuch BE et al (1997) Gene therapy of diabetes: glucose-stimulated insulin secretion in a human hepatoma cell line. Gene Ther 4:1202–1215 [DOI] [PubMed]
- 27.Fodor A, Harel C, Fodor L et al (2007) Adult rat liver cells transdifferentiated with lentiviral IPF1 vectors reverse diabetes in mice: an ex vivo gene therapy approach. Diabetologia 50:121–130 [DOI] [PubMed]
- 28.Wang AY, Ehrhardt A, Xu H, Kay MA (2007) Adenovirus transduction is required for the correction of diabetes using Pdx-1 or neurogenin-3 in the liver. Mol Ther 15:255–263 [DOI] [PubMed]
- 29.Drejer K, Kruse V, Larsen DU, Hougaard P, Bjorn S, Gammeltoft S (1991) Receptor binding and tyrosine kinase activity by insulin analogues with extreme affinities studied in human hepatoma HepG2 cells. Diabetes 40:1488–1495 [DOI] [PubMed]
- 30.Vincent MT, Carroll RJ, Hammer RE et al (1995) A transgene coding for a human insulin analog has a mitogenic effect on murine embryonic β cells. Proc Natl Acad Sci U S A 92:6239–6243 [DOI] [PMC free article] [PubMed]
- 31.Bornfeldt KE, Gidlof RA, Wateson A, Lake M, Skottner A, Arnqvist HJ (1991) Binding and biological effects of insulin, insulin analogues and insulin-like growth factors in rat aortic smooth muscle cells. Comparison of maximal growth promoting activities. Diabetologia 34:307–313 [DOI] [PubMed]
- 32.Milazzo G, Sciacca L, Papa V, Goldfine ID, Vigneri R (1997) ASPB10 insulin induction of increased mitogenic responses and phenotypic changes in human breast epithelial cells; evidence for enhanced interactions with the insulin-like growth factor-1 receptor. Mol Carcin 18:19–25 [DOI] [PubMed]
- 33.Jonsson J, Carlsson L, Edlund T, Edlund H (1994) Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371:606–609 [DOI] [PubMed]
- 34.Horb ME, Shen CN, Tosh D, Slack JM (2003) Experimental conversion of liver to pancreas. Curr Biol 13:105–115 [DOI] [PubMed]
- 35.Rao MS, Reddy JK (1995) Hepatic transdifferentiation in the pancreas. Semin Cell Biol 6:151–156 [DOI] [PubMed]
- 36.Deutsch G, Jung J, Zheng M, Lora J, Zaret KS (2001) A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128:871–881 [DOI] [PubMed]
- 37.Shanmukhappa K, Mourya R, Sabla GE, Degen JL, Bezerra JA (2005) Hepatic to pancreatic switch defines a role for hemostatic factors in cellular plasticity in mice. Proc Natl Acad Sci U S A 102:10182–10187 [DOI] [PMC free article] [PubMed]
- 38.Park I-S, Bendayan M (1993) Development of the endocrine cells in the rat pancreatic and bile duct system. Histochem J 25:807–820 [PubMed]
- 39.Cao LZ, Tang DQ, Horb ME, Li SW, Yang LJ (2004) High glucose is necessary for complete maturation of PDX1-VP16-expressing hepatic cells into functional insulin-producing cells. Diabetes 53:3168–3178 [DOI] [PMC free article] [PubMed]
- 40.Tang DQ, Cao LZ, Burkhardt BR et al (2004). In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes 53:1721–1732 [DOI] [PMC free article] [PubMed]
- 41.Ryan EA, Lakey JR, Paty BW et al (2002) Successful islet transplantation. Continued insulin reverse provides long-term glycemic control. Diabetes 51:2148–2157 [DOI] [PubMed]
- 42.Barry SC, Ramesh N, Lejnieks D et al (2001) Glucose-regulated insulin expression in diabetic rats. Human Gene Ther 12:131–139 [DOI] [PubMed]
- 43.Auricchio A, Gao GP, Yu QC et al (2002) Constitutive and regulated expression of processed insulin following in vivo hepatic gene transfer. Gene Ther 9:963–971 [DOI] [PubMed]
- 44.Zahler MH, Trani A, Mahli H et al (2000) The application of a lentiviral vector for gene transfer in fetal hepatocytes. J Gene Med 2:186–193 [DOI] [PubMed]
- 45.Berry MN, Friend DS (1969) High-yield preparation of isolated rat liver parenchymal cells. J Cell Biol 43:506–520 [DOI] [PMC free article] [PubMed]
- 46.Podevin G, Otta E, Nguyen JM et al (2004) Factors influencing immune response after in vivo retrovirus-mediated gene transfer to the liver. J Gene Med 6:16–21 [DOI] [PubMed]
- 47.deRoos WK, Fallaux FJ, Marinelli AWKS et al (1997) Isolated-organ perfusion for local gene delivery: efficient adenovirus-mediated gene transfer into the liver. Gene Ther 4:55–62 [DOI] [PubMed]
- 48.Carbonaro DA, Jin X, Petersen D et al (2006) In vivo transduction of a lentiviral vector expressing human ADA into neonatal ADA gene knockout mice: a novel form of enzyme replacement therapy for ADA deficiency. Mol Ther 13:1110–1120 [DOI] [PubMed]
- 49.Skarsgard ED, Huang L, Reebye SC, Yeung AY, Jia WW (2005) Lentiviral-mediated, in vivo gene transfer to the tracheobronchial tree in fetal rabbits. J Pediatr Surg 40:1817–1821 [DOI] [PubMed]
- 50.Nguyen TH, Oberholzer J, Birraux J, Majno P, Morel P, Trono D (2002) Highly efficient lentiviral-mediated transduction of nondividing, fully reimplantable primary hepatocytes. Mol Ther 6:199–209 [DOI] [PubMed]


