Abstract
Influx of Ca2+ ions through α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors contributes to neuronal damage in stroke, epilepsy, and neurodegenerative disorders such as ALS. The Ca2+ permeability of AMPA receptors is largely determined by the glutamate receptor 2 (GluR2) subunit, receptors lacking GluR2 being permeable to Ca2+ ions. We identified a difference in GluR2 expression in motor neurons from two rat strains, resulting in a difference in vulnerability to AMPA receptor-mediated excitotoxicity both in vitro and in vivo. Astrocytes from the ventral spinal cord were found to mediate this difference in GluR2 expression in motor neurons. The presence of ALS-causing mutant superoxide dismutase 1 in astrocytes abolished their GluR2-regulating capacity and thus affected motor neuron vulnerability to AMPA receptor-mediated excitotoxicity. These results reveal a mechanism through which astrocytes influence neuronal functioning in health and disease.
Keywords: amyotrophic lateral sclerosis, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor, neurodegeneration, mutant superoxide dismutase 1
Excessive stimulation of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)-type glutamate receptors is toxic to neurons and is known to cause neuronal death in stroke, trauma, epilepsy, and neurodegenerative disorders (1, 2). This neurotoxicity is mediated by Ca2+ entering the cell through Ca2+-permeable AMPA receptors. The Ca2+ permeability of the AMPA receptor is determined by the absence or presence of the GluR2 subunit in the receptor complex: receptors containing GluR2 have a very low relative Ca2+ permeability compared with GluR2-lacking receptor channels (3). The change of a neutral glutamine (Q) into a positively charged arginine (R) at the Q/R site of the GluR2 pre-mRNA via posttranscriptional editing is responsible for the low Ca2+ permeability of GluR2-containing AMPA receptor (4).
Low GluR2 expression yielding Ca2+-permeable AMPA receptors contributes to neuronal degeneration in ischemia (5), status epilepticus (6), and neurodegenerative disorders, such as motor neuron degeneration (7–10). On the other hand, increasing GluR2 levels or selective blockage of Ca2+-permeable AMPA receptors protects against neurodegeneration (6, 9, 11–14). Therefore, a better understanding of the regulation of GluR2 expression could lead to previously unrecognized therapeutic strategies against neuronal degeneration.
Motor neurons are thought to be particularly vulnerable to excessive AMPA receptor stimulation because of the presence of a high number of GluR2-lacking AMPA receptors (7, 9, 12). Motor neurons in culture gradually express AMPA receptors, and the presence of astrocytes is required for this differentiation (15). Low GluR2 levels yielding AMPA receptors with a high Ca2+ permeability was, however, not observed in all studies (16, 17), possibly because of the use of experimental models with a different genetic background.
In the present study, we identified a difference in GluR2 expression in motor neurons from two rat strains, which correlated with a difference in vulnerability to AMPA receptor-mediated excitotoxicity, both in vitro and in vivo. This strain difference was used to study the regulation of GluR2 expression. We discovered that astrocytes regulate this expression in a region-specific way, and the presence of mutant superoxide dismutase 1 (mt SOD1) in these cells abolished their GluR2-regulating capacity.
Results
Lower GluR2 Expression Results in Higher Vulnerability of Motor Neurons in Vitro and in Vivo.
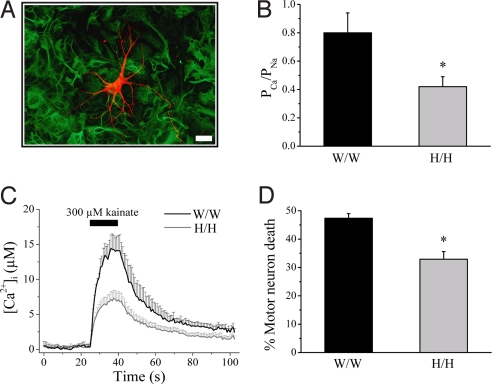
Motor neurons from two rat strains, Wistar and Holtzman, were cultured on a preestablished feeder layer of astrocytes derived from the ventral part of the spinal cord from their own strain (Fig. 1A). The expression of functional GluR2 protein in cultured motor neurons was measured by studying GluR2-dependent properties in perforated patch-clamp recordings. Compared with Holtzman motor neurons, Wistar motor neurons had AMPA receptors with a higher relative Ca2+ permeability (PCa/PNa) (Fig. 1B), a lower rectification index, and a higher sensitivity to inhibition by the polyamine 1-naphthyl acetyl spermine (also known as NAS; data not shown). This resulted in a higher elevation of the intracellular Ca2+ concentration on AMPA receptor stimulation (Fig. 1C), which correlated with a higher vulnerability of Wistar motor neurons to kainate-induced AMPA receptor-mediated excitotoxicity (Fig. 1D).
Fig. 1.
Wistar motor neurons have AMPA receptors with a higher Ca2+ permeability and are more vulnerable to excitotoxicity. (A) Coculture of motor neurons (SMI32; red) grown on a preestablished monolayer of astrocytes (GFAP; green). (Scale bar, 20 μm.) (B) PCa/PNa values of AMPA receptor currents in motor neurons when cultured on astrocytes derived from the same rat strain (W/W, Wistar motor neurons on Wistar astrocytes; H/H, Holtzman motor neurons on Holtzman astrocytes; n = 20–23; *, P = 0.017). (C) Ca2+ transients measured with the low-affinity dye Indo-1FF and induced by application of 300 μM kainate in the presence of 100 μM verapamil in cultured Wistar motor neurons (n = 19) compared with Holtzman motor neurons (n = 18; *, P = 0.014). (D) Vulnerability of cultured Wistar and Holtzman motor neurons to AMPA receptor-mediated excitotoxicity induced by a short (30-min) exposure to 300 μM kainate (n = 12–14; *, P = 0.0001).
An increased number of AMPA receptors present on the cell surface could also result in higher rises in intracellular Ca2+ concentration. However, the number of AMPA receptors present on the cell surface of motor neurons, as measured by AMPA receptor current density, was comparable for both strains (10.4 ± 1.2 and 10.1 ± 1.5 pA/pF for Wistar and Holtzman motor neurons, respectively; n = 10; P = 0.9). Furthermore, no differences in the number of synapses between both strains were observed, as assessed by counting synaptophysin-positive puncta [supporting information (SI) Fig. 6].
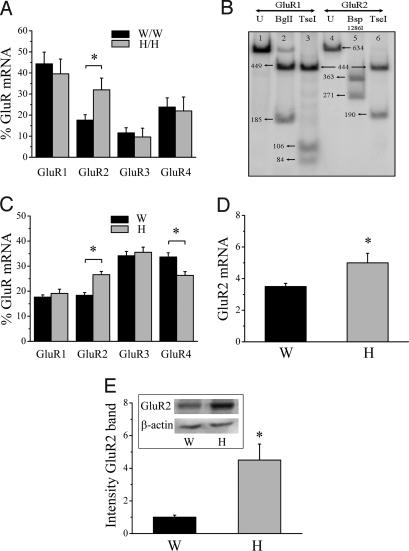
To investigate whether this lower GluR2 abundance in AMPA receptors from Wistar motor neurons was due to alterations in transcription, translation, or trafficking of the GluR2 subunit, we studied the relative mRNA expression of GluR1-4 by using single-cell RT-PCR (SI Fig. 7). Compared with Holtzman motor neurons, Wistar motor neurons had significantly lower relative GluR2 mRNA levels, which were compensated for by a slightly higher relative expression of the three other subunits (Fig. 2A). As the editing efficiency of GluR2 pre-mRNA approximated 100% in motor neurons from both strains (Fig. 2B), the difference in Ca2+ permeability observed is due to differences in GluR2 mRNA levels.
Fig. 2.
GluR2 mRNA and protein expression in Wistar and Holtzman. (A) Relative GluR mRNA expression in cultured Wistar and Holtzman motor neurons determined by single-cell RT-PCR (n = 14–18; *, P = 0.018). (B) Example of analysis of editing efficiency of GluR2 in a single-cell sample from a Wistar motor neuron. Lane 1, Undigested (U) PCR fragment of PCR specific for GluR1; lane 2, same fragment as in lane 1, digested with BglI (cleaves only GluR1 fragment); lane 3, same fragment as in lane 1, digested with TseI (which cleaves the 190-bp band into two smaller bands when not edited); thus, GluR1 is completely unedited; lane 4, undigested PCR fragment of PCR specific for GluR2; lane 5, same fragment as in lane 4, digested with Bsp 1286I (cleaves only GluR2 fragment); lane 6, same fragment as in 4, digested with TseI (which only cleaves the 190-bp band when not edited). Thus, GluR2 is completely edited. (C) Relative GluR mRNA expression in the ventral spinal cord from Wistar and Holtzman rats determined by RT-PCR (n = 5–7; *, P ≤ 0.01). (D) Real-time PCR for GluR2 normalized to 18S RNA with SYBR green on cDNA prepared from the ventral part of the spinal cord of Wistar (W) and Holtzman (H) rats (n = 8; *, P = 0.04). (E) Western blot of GluR2 in the ventral part of the spinal cord from Wistar and Holtzman rats (n = 14–16; P = 0.002). Equal loading was demonstrated by β-actin staining, and the intensities of bands were normalized to the β-actin signal.
The difference in GluR2 expression appeared to be motor neuron-specific because no differences in AMPA receptor Ca2+ permeability or relative GluR2 mRNA expression were found between cultured dorsal horn neurons from both rat strains: PCa/PNa values were 0.36 ± 0.07 and 0.36 ± 0.06 for Wistar and Holtzman, respectively (n = 13–16; P = 0.94) and relative GluR2 mRNA levels were 50.8 ± 5.3% and 46.8 ± 7.3% for Wistar and Holtzman, respectively (n = 11; P = 0.67). The relative GluR2 mRNA expression was higher in dorsal horn neurons than in motor neurons from Wistar rats (P < 0.0001) in contrast to the situation in Holtzman (P = 0.1).
The in vitro differences in GluR2 expression were also present in vivo. RT-PCR on spinal cord lysates showed a lower relative GluR2 mRNA expression in ventral spinal cords from Wistar rats compared with Holtzman rats (Fig. 2C), which was compensated for by a higher GluR4 expression. The difference in GluR2 expression in the ventral spinal cord between the two rat strains was confirmed by using real-time PCR (Fig. 2D). GluR2 immunoblot (Fig. 2E) and immunohistochemical analysis (SI Fig. 8 A–F) revealed that GluR2 protein expression was lower in ventral spinal cords from Wistar rats. GluR2 protein and mRNA expression in the dorsal horn of the spinal cord was not different between both strains (results not shown). Other synaptic proteins, such as synaptophysin, PSD95, and GRIP, were not different between both strains (SI Fig. 8 G and H).
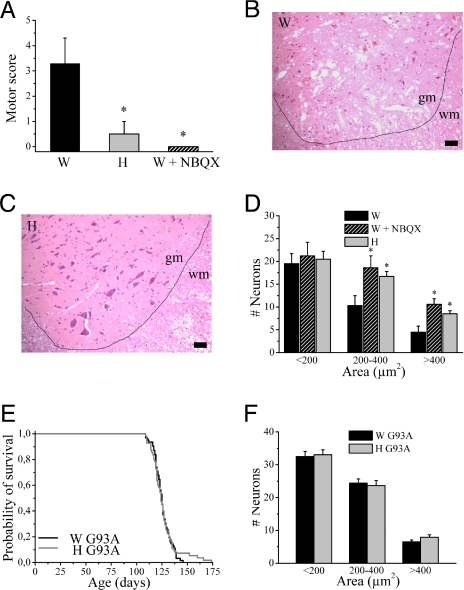
To elucidate whether this decreased GluR2 expression in Wistar spinal cord resulted in an increased vulnerability of Wistar motor neurons to AMPA receptor-mediated excitotoxicity in vivo, transient spinal cord ischemia was studied. Clamping of the aortic arch and left subclavian artery (18) resulted in a motor deficit in the hindlimbs. The extent of paresis (Fig. 3A) and motor neuron degeneration induced by ischemia was more pronounced in Wistar compared with Holtzman rats (Fig. 3 B–D). Hindlimb paresis (Fig. 3A) and motor neuron death (Fig. 3D) were mediated by AMPA receptor stimulation because they were prevented by pretreatment with the AMPA receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX).
Fig. 3.
Differences in vulnerability to excitotoxicity between both rat strains in vivo. (A) Motor score after spinal cord ischemia [0 = normal, 6 = complete paraplegia (18); n = 6–8; *, P < 0.05, different from Wistar]. (B and C) H&E staining of spinal cord section after spinal cord ischemia induced by clamping of the aortic arch and left subclavian artery for 14 min from a Wistar rat (B) and a Holtzman rat (C). gm, gray matter; wm, white matter. (Scale bar, 50 μm.) Quantification of number of neurons (divided into three size categories) in the ventral horn of the lumbar spinal cord after spinal cord ischemia, with or without pretreatment with the AMPA receptor antagonist, NBQX (n = 4–8; *, P < 0.05). Survival of Wistar and Holtzman mt SOD1 rats (n = 55–62 per group). Quantification of number of neurons (divided into three size categories) in the ventral horn of the lumbar spinal cord of end-stage Wistar and Holtzman mt SOD1 rats (n = 3; P > 0.3).
To further investigate the in vivo significance of these differences, we studied another model of motor neuron degeneration in which AMPA receptor-mediated excitotoxicity is involved, i.e., motor neuron death induced by mt SOD1. Mutations in SOD1 are known to cause familial ALS, a fatal degenerative disorder of motor neurons in humans. We studied motor neuron degeneration and survival in Wistar and Holtzman rats overexpressing mt SOD1G93A, a model for mt SOD1-induced ALS (19). Despite the known contribution of AMPA receptor stimulation (20–22) and of low GluR2 levels to mt SOD1-induced motor neuron degeneration (9, 14), no differences were found in the survival of mt SOD1 rats, irrespective of their genetic background. The average survival (±SEM) was 125.5 ± 1.0 and 126.3 ± 1.7 days for Wistar and Holtzman mt SOD1 rats, respectively (n = 55–62; P = 0.78) (Fig. 3E). Motor neuron counts in the ventral spinal cord revealed that there was no difference in motor neuron death between Wistar and Holtzman mt SOD1 rats (Fig. 3F). Although the changes in GluR2 expression that influenced motor neuron degeneration in mt SOD1 mice (9, 14) were larger than the differences observed here, the lack of protection against mt SOD1-induced motor neuron degeneration in Holtzman rats prompted further studies into the effect of mt SOD1 on the expression of GluR2 (see below).
Astrocytes Regulate GluR2 Expression.
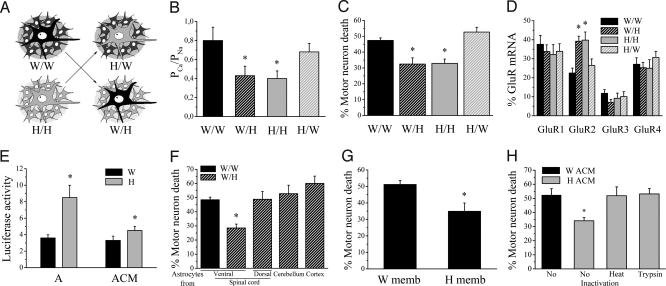
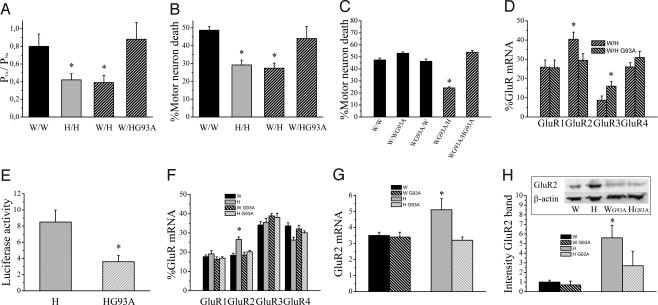
To elucidate the mechanism of differential GluR2 expression, we first sequenced the GluR2 coding sequence and promoter (3,285 bp upstream of the start codon), but no strain-specific polymorphisms could be identified (data not shown). To explore the possibility that the expression of GluR2 was regulated by astrocytes, we seeded Wistar motor neurons on Holtzman astrocytes and vice versa (Fig. 4A). Astrocytes were found to determine neuronal GluR2 expression levels. Wistar motor neurons grown on Holtzman astrocytes displayed a lower PCa/PNa than Holtzman motor neurons grown on Wistar astrocytes (Fig. 4B). Similarly, AMPA receptor currents from Wistar motor neurons grown on Holtzman astrocytes had a higher rectification index and a lower 1-naphthyl acetyl spermine sensitivity (data not shown). Cell death experiments confirmed that astrocytes determined the vulnerability to AMPA receptor-mediated excitotoxicity: Wistar motor neurons became more resistant and Holtzman motor neurons became more vulnerable to excessive AMPA receptor stimulation when cultured on astrocytes from the other strain (Fig. 4C). This astrocyte-induced switch in sensitivity to excitotoxicity was due to changes in GluR2 mRNA levels, as demonstrated by single-cell RT-PCR experiments (Fig. 4D). This result was confirmed in a GluR2 promoter reporter study. A reporter construct in which the firefly luciferase was driven by the GluR2 promoter (1,357 bp upstream of the start codon) was transfected in rat cortical neurons. An increase in luciferase activity was observed after culturing transfected neurons on top of Holtzman astrocytes (Fig. 4E).
Fig. 4.
Astrocytes regulate GluR2 expression in motor neurons. (A) Schematic representation of the different motor neuron and astrocyte combinations. W/W, Wistar motor neurons on Wistar astrocytes; H/H, Holtzman motor neurons on Holtzman astrocytes; W/H, Wistar motor neurons on Holtzman astrocytes; H/W, Holtzman motor neurons on Wistar astrocytes. (B) Functional determination of the GluR2 expression in motor neurons when cultured on astrocytes from the same and the other rat strain. The PCa/PNa values of AMPA receptor currents are shown (n = 21–23, W/W and H/W not different; *, P < 0.05, significantly different from W/W and H/W). (C) Kainate-induced AMPA receptor-mediated excitotoxicity in W/W, W/H, H/H, and H/W (n = 4–7, W/W and H/W not different; *, P ≤ 0.005, significantly different from W/W and H/W). (D) Relative GluR mRNA expression determined by single-cell RT-PCR in W/W, W/H, H/H, and H/W (n = 21–26; *, P ≤ 0.03, significantly different from neurons cultured on Wistar astrocytes). (E) Effect of presence of astrocytes (A) or ACM (added directly after seeding, for 48 h) on luciferase activity in cortical neurons (normalized to β-galactosidase activity and background activity of empty vector; n = 5–10; *, P < 0.05). (F) AMPA receptor-mediated excitotoxicity in Wistar motor neurons grown on Holtzman astrocytes from different regions. VSC, ventral spinal cord; DSC, dorsal spinal cord; CER, cerebellum; CTX, cortex. n = 3–7; *, P < 0.025, different from other groups. (G) AMPA receptor-mediated excitotoxicity in W/W in the presence of astrocytic membrane fractions prepared from Wistar or Holtzman astrocytes (n = 3; P = 0.03). (H) AMPA receptor-mediated excitotoxicity in W/W in the presence of ACM from Wistar or Holtzman astrocytes, with or without pretreatment with heat or trypsin (n = 4; *, P < 0.015, significantly different from other groups).
We investigated the specificity of the GluR2-regulating capacity of Holtzman astrocytes. We first explored whether Holtzman astrocytes also affected properties of other synaptic currents (such as GABA and glycine receptor currents) but found no effect (data not shown). Furthermore, we discovered that the Holtzman astrocyte-induced protection against excitotoxicity was regionally specific, because it was only apparent for astrocytes derived from the ventral part of the spinal cord, and not for astrocytes cultured from the dorsal spinal cord, the cerebellum, or the cortex (Fig. 4F).
We further characterized the nature of the astrocytic factor regulating GluR2 expression. The GluR2-regulating factor(s) were present in both astrocyte-conditioned medium (ACM) and astrocytic membrane fractions. Holtzman ACM stimulated luciferase activity, albeit to a lesser degree than when cortical neurons were grown in close contact with Holtzman astrocytes (Fig. 4E). Furthermore, pretreatment of motor neuron cultures with both Holtzman astrocytic membrane fractions (Fig. 4G) and Holtzman ACM (Fig. 4H) gave rise to protection against excitotoxicity, suggesting that the factor(s) are released by astrocytes but are also present in membranous fractions. Heat inactivation and protease treatment of Holtzman ACM abolished its capability of improving motor neuron survival, indicating that the factor(s) mediating the effect are proteinaceous in nature (Fig. 4H).
mt SOD1 Abolishes the Astrocytic Up-Regulation of GluR2 Expression.
In view of the lack of difference in survival between Wistar and Holtzman mt SOD1 rats, we studied in vitro the effect of mt SOD1 on the astrocytic up-regulation of GluR2 expression. We discovered that the presence of mt SOD1 in Holtzman astrocytes abolished their ability to decrease the Ca2+ permeability of AMPA receptor currents in motor neurons (Fig. 5A) and, likewise, to protect them against AMPA receptor-mediated excitotoxicity (Fig. 5B). The presence of mt SOD1 in neurons did not influence their vulnerability to excitotoxicity and did not affect their response to the protective factor(s) derived from Holtzman astrocytes (Fig. 5C). Single-cell RT-PCR experiments showed that Holtzman astrocytes containing mt SOD1 were no longer able to increase the relative GluR2 mRNA expression in motor neurons (Fig. 5D). Luciferase experiments confirmed that the presence of mt SOD1 in Holtzman astrocytes abolished their capacity to stimulate GluR2 expression (Fig. 5E). Similar observations were made in vivo. Measurement of the relative abundance of the four GluR subunits by RT-PCR showed that the elevated relative GluR2 mRNA content in the ventral spinal cord from Holtzman rats was absent in mt SOD1 animals (Fig. 5F). The lower GluR2 mRNA level in mt SOD1 Holtzman rats was confirmed by real-time PCR (Fig. 5G). At the protein level, the elevated GluR2 expression in the ventral spinal cord from Holtzman rats was largely reduced in mt SOD1 animals (Fig. 5H).
Fig. 5.
mt SOD1 in astrocytes abolishes their GluR2-regulating capacity. (A) PCa/PNa values of AMPA receptor currents in W/W, H/H, W/H, and W/H (n = 11–23; *, P < 0.03). (B) AMPA receptor-mediated excitotoxicity in W/W, H/H, W/H, and W/H (n = 5–18; *, P < 0.04). (C) AMPA receptor-mediated excitotoxicity in Wistar motor neurons with or without mt SOD1 grown on different combinations of astrocytes with or without mt SOD1 (n = 4–9; *, P ≤ 0.01, significantly different from all other groups). (D) Relative GluR mRNA expression in W/H and W/HG93A (n = 14–17; *, P < 0.04). (E) Effect of presence of mt SOD1 in Holtzman astrocytes on luciferase activity in cortical neurons seeded on Holtzman astrocytes (normalized to β-galactosidase activity and background activity of empty vector, n = 6–8; *, P = 0.015). (F) Relative GluR mRNA expression in the ventral spinal cord from Wistar and Holtzman rats with or without mt SOD1 determined by RT-PCR (n = 5–7; *, P ≤ 0.01, significantly different from other groups). (G) Real-time PCR for GluR2 normalized to 18S RNA with SYBR green on cDNA prepared from the ventral part of the spinal cord of Wistar and Holtzman rats with or without mt SOD1 (n = 8–10; *, P < 0.04, significantly different from other groups). (H) Western blot of GluR2 in the ventral part of the spinal cord from Wistar and Holtzman rats with or without mt SOD1 (n = 8–11; *, P < 0.05). Equal loading was demonstrated by β-actin staining, and the intensity of bands was normalized to the β-actin signal.
Together, these data suggest that the deleterious effect of mt SOD1 on the GluR2-up-regulating capacity of astrocytes is also at play in vivo and most likely contributes to the lack of difference in survival between mt SOD1 Wistar and Holtzman rats mentioned above.
Discussion
In the last few decades, it became evident that astrocytes are not merely electrically silent supporting cells. They appear to be essential to normal brain functioning, and intensive interactions between astrocytes and neurons were discovered (23). Astrocytes are organized in communicating networks, have rapid intercellular communication, secrete neurotrophic and other factors that affect neuronal functioning, and take part in the removal of neurotransmitters from the synaptic cleft. The present study adds to this growing list of neuron–glia interactions a role for astrocytes in regulating neuronal GluR2 expression, an AMPA receptor subunit that mediates vulnerability of neurons to excitotoxic cell death.
A low expression of the AMPA receptor subunit GluR2, which results in the presence of AMPA receptors with a high Ca2+ permeability, is of importance to neurodegeneration in several models of AMPA receptor-mediated excitotoxicity. The degeneration of hippocampal pyramidal neurons after transient global ischemia or status epilepticus is preceded by GluR2 down-regulation, and the presence of GluR2-lacking AMPA receptors has been shown to be sufficient to induce neurodegeneration in these brain areas (5, 6, 24). For motor neurons, a down-regulation of GluR2 expression preceding neurodegeneration has never been observed, but a constitutive low, relative GluR2 expression is likely to contribute to the selective vulnerability of motor neurons to AMPA receptor stimulation (7, 8, 25), and to the selective motor neuron degeneration in mt SOD1 mice (9, 14), a transgenic animal model for familial ALS. In the present study, we found a strain-specific difference in vulnerability of spinal motor neurons to excessive AMPA receptor stimulation both in vitro and in vivo, which correlated with a difference in relative GluR2 expression. Lower GluR2 mRNA levels were found in cultured motor neurons and in the ventral spinal cord from Wistar rats. At the protein level, lower GluR2 protein levels were observed in Wistar ventral spinal cord. Although GluR2 immunopositivity is mainly observed in large neurons in the ventral horn of the spinal cord, it is possible that changes in GluR2 expression in other cell types than motor neurons contribute to the difference observed. The contradiction between studies that claimed motor neurons to have an average (16, 17) or low GluR2 expression (7) can be explained on the basis of the rat strain used.
The difference in GluR2 expression was already observed at the mRNA level, pointing toward differences in transcription, rather than changes in GluR2 translation, trafficking, or delivery of GluR2 from surroundings cells by exosomes (26). However, additional regulation of GluR2 expression at the posttranscriptional level cannot be excluded, because the difference in GluR2 was more pronounced at the protein level than at the mRNA level. We excluded that the difference in GluR2 transcription between both rat strains was due to changes in the GluR2 promoter. The relative expression of the three other AMPA receptor subunits varied considerably between the in vitro and in vivo situation, in contrast to GluR2 levels, suggesting that the relative expression of GluR2 mRNA is tightly regulated.
Our culture model of purified motor neurons grown on a preestablished astrocytic monolayer allowed us to determine whether the mechanism that modulates GluR2 expression is motor neuron-autonomous or rather depends on surrounding astrocytes. Exchanging the astrocytic feeder layer reversed the differences between Wistar and Holtzman motor neurons. This indicates that factors derived from astrocytes regulate GluR2 expression in motor neurons.
The origin of the astrocytes appeared critical to this regulation, because only astrocytes derived from the ventral spinal cord of Holtzman rats were able to induce an up-regulation of GluR2 expression. The lack of GluR2 up-regulation induced by Holtzman astrocytes from the dorsal spinal cord, the cerebellum, and the cortex are in agreement with previous evidence that astrocytes are organized in region-specific territories (27) with different characteristics and gene expression profiles (28). Further characterization of the astrocytic factor(s) involved, revealed that both ACM and membrane fractions were able to stimulate GluR2 expression in motor neurons. This suggests that the GluR2-up-regulating factor may be released from the plasma membrane or that more than one factor is involved. The factor(s) were found to be proteinaceous in nature.
Our results further showed that this astrocyte–neuronal interaction was also relevant to the mechanism of mt SOD1-induced motor neuron death. In this type of motor neuron degeneration, AMPA receptor-mediated excitotoxicity caused by Ca2+ influx through GluR2-lacking AMPA receptors is known to play a role (29). We have previously shown that deleting the GluR2 gene accelerates motor neuron degeneration in mt SOD1 mice (9), whereas overexpression of GluR2 was found to attenuate the disease (14).
mt SOD1-induced motor neuron degeneration in vivo was not different between the two rat strains, despite the difference in GluR2 expression. This was due to the fact that the presence of mt SOD1 abolished the astrocytic regulation of GluR2 expression both in vitro and in vivo. Both at the mRNA and the protein level, the higher GluR2 expression observed in Holtzman ventral spinal cords was attenuated in Holtzman mt SOD1 animals. In Wistar ventral spinal cords, a very small reduction in GluR2 protein levels (which did not reach significance) was observed in mt SOD1 animals, without a change in GluR2 mRNA levels. A similar decrease in GluR2 protein levels without changes in GluR2 mRNA was recently observed in mt SOD1 murine spinal motor neurons (22).
Regulating neuronal GluR2 expression seems to be a second mechanism for astrocytes to regulate excitotoxic neuronal death, in addition to the reuptake of glutamate by the glial glutamate transporters (30, 31). Disturbances in both processes may contribute to the motor neuron degeneration in ALS. In particular, they favor the concept of the non-cell-autonomous character of motor neuron death in this disease (32). Toxic factors released from glial cells expressing mt SOD1 have recently been demonstrated to contribute to the motor neuron toxicity of mt SOD1 (33–35). Here, we found evidence for protective factors released from astrocytes, but the protective effects were abolished when mt SOD1 was present in astrocytes.
In conclusion, our findings show that the interplay between astrocytes and neurons is paramount to neuronal functioning and that disturbances in this intercellular communication may contribute to the pathogenesis of motor neuron degeneration. Further studies are needed to identify astrocytic factors that alter neuronal vulnerability.
Experimental Procedures
Cell Cultures, Cell Death Experiments, Ca2+ Measurements, and Perforated Patch-Clamp Recordings.
Motor neurons were cultured on a preestablished feeder layer of astrocytes as described in ref. 12. The purity of motor neurons in culture and of the astrocytic feeder layer was demonstrated by immunocytochemistry (see SI Experimental Procedures). Cell death experiments (36), Ca2+ measurements (37), and perforated patch-clamp recordings (7) were performed as described previously (see also SI Experimental Procedures).
RT-PCR.
RT-PCR experiments were performed on single cells aspirated through the patch pipette and on RNA samples derived from ventral spinal cord lysates. Single-cell RT-PCR experiments were carried out as described in refs. 17 and 38 with minor modifications (see SI Experimental Procedures; SI Fig. 7 shows the validation of the technique).
Real-Time PCR.
RNA samples from Wistar and Holtzman ventral spinal cord were subjected to real-time quantitative PCR using SYBR green I on a 7000 Sequence Detection system (Applied Biosystems, Foster City, CA) and normalized to 18S RNA (see also SI Experimental Procedures).
Spinal Cord Ischemia.
Wistar and Holtzman female rats 4–5 months of age were subjected to spinal cord ischemia as described in ref. 18. A clinical score (0, no deficit; 6, maximal deficit) was used to estimated the deficit (18), and motor neuron survival in the ventral spinal cord was quantified 24 h after the surgery.
mt SOD1 Rats.
Rats overexpressing human mt SOD1G93A (in a Sprague–Dawley background) were provided by D. Howland (Wyeth Research, Princeton, NJ) (19) and backcrossed for 6–12 generations into the Wistar or Holtzman genetic background. In survival experiments, the time of death was defined as the day when the rats had lost 40% of their maximal presymptomatic weight. For Western blot, RT-PCR, and real-time PCR experiments on spinal cords, presymptomatic animals (≈40 days of age) were used.
Sequencing.
Exon 1, intron 1, a 3,285-bp genomic sequence upstream of the GluR2 start codon, and cDNA were sequenced in three different Wistar and Holtzman rats (see SI Experimental Procedures).
Western Blot.
Spinal cords from Wistar and Holtzman rats were isolated, separated into ventral and dorsal parts, and homogenized in radioimmunoprecipitation assay buffer (also known as RIPA) as described in ref. 39. More details are provided in SI Experimental Procedures.
Luciferase Assay.
A 1,357-bp fragment of the GluR2 promoter was cloned upstream of the luciferase gene in a pGL3-basic vector (Promega, Madison, WI). Luciferase activity was measured in cell lysates of transfected cortical neurons as described in SI Experimental Procedures.
Materials and Statistics.
A list of all chemicals is provided in SI Experimental Procedures.
All experiments and procedures were approved by the Ethical Committee of the Katholieke Universiteit Leuven.
Average data are shown as the mean ± SEM. Student's t tests were used to calculate significance when two groups were compared. When more than two groups were compared, a one-way ANOVA was used and a Bonferroni comparison was used to compare between the different groups. For data without a Gaussian distribution, significance was calculated by using nonparametric statistics (Mann–Whitney U test). For luciferase experiments, binomial statistics were used.
Supplementary Material
Acknowledgments
We thank Dr. J. Brorson (University of Chicago, Chicago, IL) for providing us with GluR1–4 plasmids. This work was supported by grants from the Fund for Scientific Research Flanders, the University of Leuven, the Belgian government (Interuniversity Attraction Poles, Program P6/43 of the Belgian Federal Science Policy Office), the Stem Cell Institute Leuven, and the ALS Association. E.B. is a research assistant and P.V.D. is a postdoctoral fellow of the Fund for Scientific Research Flanders. W.R. is supported through the E. von Behring Chair for Neuromuscular and Neurodegenerative Disorders.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline
- mt SOD1
mutant superoxide dismutase 1
- ALS
amyotrophic lateral sclerosis
- ACM
astrocyte-conditioned medium.
Footnotes
This article is a PNAS Direct Submission.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705046104/DC1.
References
- 1.Lipton SA, Rosenberg PA. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 2.Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W. Biochim Biophys Acta. 2006;1762:1068–1082. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Hollmann M, Hartley M, Heinemann S. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 4.Sommer B, Kohler M, Sprengel R, Seeburg PH. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 5.Gorter JA, Petrozzino JJ, Aronica EM, Rosenbaum DM, Opitz T, Bennett MV, Connor JA, Zukin RS. J Neurosci. 1997;17:6179–6188. doi: 10.1523/JNEUROSCI.17-16-06179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grooms SY, Opitz T, Bennett MV, Zukin RS. Proc Natl Acad Sci USA. 2000;97:3631–3636. doi: 10.1073/pnas.050586497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Damme P, Van Den Bosch L, Van Houtte E, Callewaert G, Robberecht W. J Neurophysiol. 2002;88:1279–1287. doi: 10.1152/jn.2002.88.3.1279. [DOI] [PubMed] [Google Scholar]
- 8.Kawahara Y, Kwak S, Sun H, Ito K, Hashida H, Aizawa H, Jeong SY, Kanazawa I. J Neurochem. 2003;85:680–689. doi: 10.1046/j.1471-4159.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Damme P, Braeken D, Callewaert G, Robberecht W, Van Den Bosch L. J Neuropathol Exp Neurol. 2005;64:605–612. doi: 10.1097/01.jnen.0000171647.09589.07. [DOI] [PubMed] [Google Scholar]
- 10.Kuner R, Groom AJ, Bresink I, Kornau HC, Stefovska V, Muller G, Hartmann B, Tschauner K, Waibel S, Ludolph AC, et al. Proc Natl Acad Sci USA. 2005;102:5826–5831. doi: 10.1073/pnas.0501316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronica EM, Gorter JA, Grooms S, Kessler JA, Bennett MV, Zukin RS, Rosenbaum DM. Proc Natl Acad Sci USA. 1998;95:7115–7120. doi: 10.1073/pnas.95.12.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Den Bosch L, Vandenberghe W, Klaassen H, Van Houtte E, Robberecht W. J Neurol Sci. 2000;180:29–34. doi: 10.1016/s0022-510x(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Calderone A, Jover T, Grooms SY, Yokota H, Zukin RS, Bennett MV. Proc Natl Acad Sci USA. 2002;99:2362–2367. doi: 10.1073/pnas.261713299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tateno M, Sadakata H, Tanaka M, Itohara S, Shin RM, Miura M, Masuda M, Aosaki T, Urushitani M, Misawa H, et al. Hum Mol Genet. 2004;13:2183–2196. doi: 10.1093/hmg/ddh246. [DOI] [PubMed] [Google Scholar]
- 15.Vandenberghe W, Van Den Bosch L, Robberecht W. Brain Res. 1998;807:1–10. doi: 10.1016/s0006-8993(98)00569-1. [DOI] [PubMed] [Google Scholar]
- 16.Greig A, Donevan SD, Mujtaba TJ, Parks TN, Rao MS. J Neurochem. 2000;74:179–191. doi: 10.1046/j.1471-4159.2000.0740179.x. [DOI] [PubMed] [Google Scholar]
- 17.Vandenberghe W, Robberecht W, Brorson JR. J Neurosci. 2000;20:123–132. doi: 10.1523/JNEUROSCI.20-01-00123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C. J Vasc Surg. 2003;38:564–575. doi: 10.1016/s0741-5214(03)00473-7. [DOI] [PubMed] [Google Scholar]
- 19.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, et al. Proc Natl Acad Sci USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canton T, Bohme GA, Boireau A, Bordier F, Mignani S, Jimonet P, Jahn G, Alavijeh M, Stygall J, Roberts S, et al. J Pharmacol Exp Ther. 2001;299:314–322. [PubMed] [Google Scholar]
- 21.Van Damme P, Leyssen M, Callewaert G, Robberecht W, Van Den Bosch L. Neurosci Lett. 2003;343:81–84. doi: 10.1016/s0304-3940(03)00314-8. [DOI] [PubMed] [Google Scholar]
- 22.Tortarolo M, Grignaschi G, Calvaresi N, Zennaro E, Spaltro G, Colovic M, Fracasso C, Guiso G, Elger B, Schneider H, et al. J Neurosci Res. 2006;83:134–146. doi: 10.1002/jnr.20715. [DOI] [PubMed] [Google Scholar]
- 23.Volterra A, Meldolesi J. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 24.Oguro K, Oguro N, Kojima T, Grooms SY, Calderone A, Zheng X, Bennett MV, Zukin RS. J Neurosci. 1999;19:9218–9227. doi: 10.1523/JNEUROSCI.19-21-09218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath PR, Tomkins J, Ince PG, Shaw PJ. NeuroReport. 2002;13:1753–1757. doi: 10.1097/00001756-200210070-00012. [DOI] [PubMed] [Google Scholar]
- 26.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. Proc Natl Acad Sci USA. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamak B, Fellous A, Glowinski J, Prochiantz A. J Neurosci. 1987;7:3163–3170. doi: 10.1523/JNEUROSCI.07-10-03163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachoo RM, Kim RS, Ligon KL, Maher EA, Brennan C, Billings N, Chan S, Li C, Rowitch DH, Wong WH, et al. Proc Natl Acad Sci USA. 2004;101:8384–8389. doi: 10.1073/pnas.0402140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleveland DW, Rothstein JD. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 30.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 31.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, et al. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 32.Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, et al. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 33.Kim YS, Martinez T, Deshpande DM, Drummond J, Provost-Javier K, Williams A, McGurk J, Maragakis N, Song H, Ming GL, et al. Ann Neurol. 2006;60:716–728. doi: 10.1002/ana.21034. [DOI] [PubMed] [Google Scholar]
- 34.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Damme P, Callewaert G, Eggermont J, Robberecht W, Van Den Bosch L. J Neurosci. 2003;23:4942–4950. doi: 10.1523/JNEUROSCI.23-12-04942.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Den Bosch L, Schwaller B, Vleminckx V, Meijers B, Stork S, Ruehlicke T, Van Houtte E, Klaassen H, Celio MR, Missiaen L, et al. Exp Neurol. 2002;174:150–161. doi: 10.1006/exnr.2001.7858. [DOI] [PubMed] [Google Scholar]
- 38.Van Den Bosch L, Verhoeven K, De Smedt H, Wuytack F, Missiaen L, Robberecht W. Life Sci. 1999;65:1597–1606. doi: 10.1016/s0024-3205(99)00405-1. [DOI] [PubMed] [Google Scholar]
- 39.Vleminckx V, Van Damme P, Goffin K, Delye H, Van Den Bosch L, Robberecht W. J Neuropathol Exp Neurol. 2002;61:968–974. doi: 10.1093/jnen/61.11.968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.