Abstract
The ability of estrogen receptor α (ERα) to modulate gene expression is influenced by the recruitment of a host of co-regulatory proteins to target genes. To further understand how estrogen-responsive genes are regulated, we have isolated and identified proteins associated with ERα when it is bound to DNA containing the consensus estrogen response element (ERE). One of the proteins identified in this complex, proliferating cell nuclear antigen (PCNA), is required for DNA replication and repair. We show that PCNA interacts with ERα in the absence and in the presence of DNA, enhances the interaction of ERα with ERE-containing DNA, and associates with endogenous estrogen-responsive genes. Interestingly, rather than altering hormone responsiveness of endogenous, estrogen-responsive genes, PCNA increases the basal expression of these genes. Our studies suggest that in addition to serving as a platform for the recruitment of DNA replication and repair proteins, PCNA may serve as a platform for transcription factors involved in regulating gene expression.
INTRODUCTION
Estrogen receptor alpha (ERα) is a ligand-activated transcription factor that alters the expression of a wide variety of estrogen-responsive genes in target cells (1,2). It is essential for development of the reproductive tract and maintenance of reproductive function (3,4).
ERα is comprised of six functional domains (A–F) that have been evolutionarily conserved (5,6). The most highly conserved region is domain C, the DNA-binding domain (DBD), which is comprised of two zinc finger domains. The DBD is necessary and sufficient for specific interaction of the receptor with its DNA recognition sequence, the estrogen response element (ERE). Domain E, the ligand-binding domain (LBD), is also highly conserved and directs the specific interaction of the receptor with hormone. In addition to these two highly conserved domains are regions with considerable variation in amino acid sequence, including the amino terminal A/B domain, the carboxy terminal F domain, and the centrally located hinge region, domain D. Sequence analysis of ERα from different species in combination with functional studies of mutant receptors have identified two regions of the receptor that are important in enhancing estrogen-responsive gene expression (7,8). The ligand-independent activation function 1, AF-1, is localized in the amino terminal A/B domain of the receptor and the hormone-inducible activation function 2, AF-2, is present in the LBD (9,10).
Upon binding hormone, ERα undergoes a conformational change, binds to EREs residing in estrogen-responsive genes, and recruits co-regulatory proteins to initiate changes in gene expression (11,12). These co-regulatory proteins include chromatin remodelers, modifiers of post-translational acetylation and phosphorylation, and an increasing number of cell-cycle and DNA repair-related factors (13–22). This extensive array of co-regulatory proteins, which possess a wide variety of functional activities, helps to ensure fine-tuned control of estrogen-responsive gene expression.
In order to identify novel co-regulatory proteins involved in ERα-mediated gene expression, we utilized a modified gel mobility shift assay to isolate proteins associated with the DNA-bound receptor and then identified the isolated proteins by mass spectrometry analysis (22,23). One protein of particular interest was proliferating cell nuclear antigen (PCNA), which is required for DNA replication and repair. Interestingly, PCNA interacts directly with the DNA repair protein flap endonuclease-1 [FEN-1 (24–26)], which we recently identified as a modulator of ERα-mediated transcription (22). In addition, PCNA has been used as an independent marker of breast, renal and skin cancer (27–30).
We have characterized the association of PCNA with ERα and find that PCNA interacts with ERα, enhances the receptor–DNA interaction in vitro, and associates with endogenous, estrogen-responsive genes. Rather than influencing estrogen responsiveness in MCF-7 breast cancer cells, PCNA helps to maintain the basal expression of estrogen-responsive genes.
MATERIALS AND METHODS
Isolation and identification of PCNA
Nuclear extracts (20 μg) from HeLa cervical cancer cells were incubated with annealed, 32P-labeled oligos containing the Xenopus laevis vitellogenin A2 ERE (5′-GAT TAA CTG TCC AAA GTC AGG TCA CAG TGA CCT GAT CAA AGT TAA TGT AA-3′ and 5′-TTA CAT TAA CTT TGA TCA GGT CAC TGT GAC CTG ACT TTG GAC AGT TAA TC-3′) in the absence or presence of 400 fmol of purified, baculovirus-expressed ERα. Incubations were performed in agarose-binding buffer (15 mM Tris pH 7.9, 56 mM KCl, 0.2 mM EDTA, 4 mM DTT, 5 mM MgOAc, 0.05 mM ZnCl2) with 10% v/v glycerol, 100 ng of poly dI/dC, 1 μg salmon sperm DNA and 10 nM 17β-estradiol (E2) in a final volume of 12.5 μl for 10 min on ice. Proteins associated with the ERE-bound ERα were separated on a 1.75% low melt agarose gel with modified TBE buffer (4.5 mM Tris pH 7.9, 44.3 mM boric acid, 5.2 mM MgOAc and 1 mM EDTA).
For large-scale isolation of protein complexes, reactions were increased 10-fold and proteins were identified using mass spectrometry analysis essentially as previously described (23). Nine discrete peptide fragments with amino acid sequence identical to that found in PCNA (LVQGSILKK, NLAMGVNLTSMSK, FSASGELGNGNIK, LMDLDVEQLGIPEQEYSCVVK, YLNFFTK, ATPLSSTVTLSMSADVPLVVEYK, DLSHIGDAVVISCAK, FSASGELGNGNIKLSQTSNVDKEEEAVTIEMNEPVQLTFALR, AEDNADTLALVFEAPNQEK) were identified in two independent experiments. These peptides comprised 57% of the total PCNA amino acid sequence. Control lanes lacking ERα were run on the agarose gels in parallel to ensure that PCNA was associated with the DNA-bound ERα and did not simply co-migrate with the receptor–DNA complex.
Expression and purification of his-tagged PCNA
A bacterial expression vector encoding his-tagged PCNA (pHKEp-PCNA) was graciously provided by Zvi Kelman [University of Maryland Biotechnology Institute, Rockville, MD, USA (31)]. Expression and purification of his-tagged PCNA was performed as previously described (23). Protein purity was assessed on Coomassie stained gels and protein concentration was determined using the BioRad protein assay (BioRad, Hercules, CA, USA) with BSA as a standard.
Pull-down assays using truncated ERα proteins
Pull-down assays using in vitro transcribed and translated 35S-labeled full-length ERα or truncated ERα proteins ABC, AB, CD and DEF (32–34) were performed essentially as described (23). Expression vectors for ▵CD1 (amino acids 180–292), ▵CD2 (amino acids 180–272) and C (amino acids 180–262) were provided by Kendall Nettles (The Scripps Institute, Jupiter, FL, USA) and synthesized as described in Stols et al. (35). Full length and truncated ERα proteins were synthesized using the TNT T7 Quick Coupled Transcription/Translation system (Promega, Madison, WI, USA) and incubated with immobilized, his-tagged PCNA. For E domain interaction studies, pET15b-ERα (304–554) [kindly provided by Benita Katzenellenbogen, University of Illinois, Urbana, IL (36)] which encoded the his-tagged E domain of ERα, was transformed into Escherichia coli, expressed and immobilized on Ni-NTA beads as previously described (23), followed by incubation with full length, untagged PCNA (a gift from John Bruning and Kendall Nettles, The Scripps Institute, Jupiter, FL, USA). Incubations were done at 4°C for 45 min in binding buffer (15 mM Tris pH 7.9, 20 mM KCl, 0.2 mM EDTA, 4 mM DTT) with or without 10 μM E2.
Bound proteins were washed once with binding buffer, once with wash buffer (15 mM Tris pH 7.9, 100 mM KCl, 0.2 mM EDTA, 4 mM DTT), and then eluted with 2 × loading buffer (125 mM Tris pH 6.8, 4% v/v SDS, 20% v/v glycerol, 1.44 M β-mercaptoethanol). Eluted proteins were separated by SDS–PAGE and subjected to autoradiography (for 35S-labeled proteins). For pulldowns using purified PCNA, eluted proteins were separated by SDS–PAGE and subjected to western blot analysis with antibodies specific for PCNA, his-tag and ERα (sc-7907, sc-803 and sc-8002, respectively, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The blots were probed with a horseradish peroxidase-conjugated secondary antibody and developed using a chemiluminescent detection system as previously described (37).
Pull-down assays using full-length proteins
Flag-tagged full-length ERα was expressed in Sf9 cells as previously described (38,39), immobilized on M2-agarose (Sigma, St Louis, MO, USA), and washed with purification buffer (20 mM Tris pH 7.5, 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA and 10% v/v glycerol). Twenty micrograms of MCF-7 nuclear extracts or purified, untagged PCNA (a gracious gift from John Bruning and Kendall Nettles, The Scripps Institute, Jupiter, FL, USA) were incubated with immobilized ERα in the absence or presence of DNA oligos containing an ERE (described above for PCNA isolation) or non-specific DNA sequence (5′-CTA GAT TAC TTC TCA TGT TAG ACA TAC TCA GAT CTA GAC ATA CTC AGA TC-3′ and 5′-GAT CTG AGT ATG TCT AGA TCT GAG TAT GTC TAA CAT GAG AAG TAA TCT AG-3′) in binding buffer, followed by washing and elution as described. Eluted proteins were separated by SDS–PAGE and subjected to western blot analysis with PCNA (sc-56 or sc-7907) or ERα (sc-8002) specific antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The blots were probed with a horseradish peroxidase-conjugated secondary antibody and developed using a chemiluminescent detection system as previously described (37).
Polyacrylamide gel mobility shift assays
ERα was expressed and purified to near homogeneity as we have previously described (38). Purified ERα measuring 10–50 fmol were incubated without or with 0.5–2.5 μg of purified his-PCNA in binding buffer with 10% v/v glycerol, 100 ng of poly dI/dC and 10 nM E2 in a final volume of 20 μl for 10 min on ice. BSA and His elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole) were included as needed to maintain constant protein and salt concentrations. For antibody supershift experiments, an ERα- or PCNA-specific antibody (sc-8002 or sc-56, respectively, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added to the binding reaction and incubated for 10 min on ice prior to addition of DNA. Radiolabeled ERE-containing oligos were added to the binding reactions and incubated for 10 min at room temperature prior to fractionation on low ionic strength polyacrylamide gels (40) at 4°C with buffer re-circulation. Radioactive bands were visualized by autoradiography or were quantitated by phosphoimager analysis with Image Quant software (GE Healthcare, Piscataway, NJ, USA).
Chromatin immunoprecipitation assays
MCF-7 cells were maintained in phenol red-containing MEM supplemented with 5% v/v calf serum, placed on phenol red-free MEM with 5% v/v CDCS for at least 72 h and exposed to ethanol vehicle or 10 nM E2 for 2 h. Chromatin immunoprecipitation assays were carried out essentially as recommended by Millipore (Charlottesville, VA, USA) except that pelleted cells were washed three times in lysis buffer (10 mM Tris, pH 7.5, 10 mM NaCl, 3 mM MgCl2) with 0.5% v/v NP-40, re-suspended in lysis buffer with 10 mM CaCl2 and 4% v/v NP-40, and treated with 75 U micrococcal nuclease (USB, Cleveland, OH, USA) for 10 min prior to sonication. The ERα- and PCNA-specific antibodies sc-8002 and sc-56, respectively (Santa Cruz Biotechnology, Santa Cruz, CA, USA), were used for immunoprecipitation of protein–DNA complexes. PCR primers (Table 1) flanking the pS2 ERE or a region 2.8-kb upstream of this site, which contains no known ERα-binding sequence, were used for quantitative PCR analysis with iQ SYBR Green Supermix and the iCycler PCR thermocycler according to manufacturer's directions for 40 amplification cycles (BioRad, Hercules, CA, USA). One thousand, 5000, 10 000 and 25 000 genomic copies were run in parallel with each primer set during each experiment to derive a standard curve. The relative copy number for each sample was determined from the standard curve. Each sample was run in triplicate and data from four independent experiments is reported as the relative number of copies of specific DNA sequence. Significant changes in induction were calculated using the student's t-test.
Table 1.
PCR primer sequences
| ChIP analysis | |
|---|---|
| pS2 ERE-containing region | 5′-CCCGTGAGCCACTGTTGTC-3′ |
| 5′-CCTCCCGCCAGGGTAAATAC-3′ | |
| pS2 control region | 5′-GTATGGTGTGGTCTTGGGTTCC-3′ |
| 5′-GGGTTGGAGCGGCTGGAG-3′ | |
| Oxytocin ERE-containing region | 5′-AAGGCACCTCACCTTCTGTG-3′ |
| 5′-TCGGTGGAGCTCTGTTTAAGA-3′ | |
| 36B4 control region | 5′-GTGTTCGACAATGGCAGCAT-3′ |
| 5′-GACACCCTCCAGGAAGCGA-3′ |
| RT-PCR analysis | |
|---|---|
| PCNA mRNA | 5′-CCTGTAGCGGCGTTGTTG-3′ |
| 5′-CGTTGATGAGGTCCTTGAGTG-3′ | |
| PR mRNA | 5′-GTGCCTATCCTGCCTCTCAATC-3′ |
| 5′-CCCGCCGTCGTAACTTTCG-3′ | |
| pS2 mRNA | 5′-GCTGTTTCGACGACACCGTT-3′ |
| 5′-TTCTGGAGGGACGTCGATG-3′ | |
| ERα mRNA | 5′-TGCCCTACTACCTGGAGAAC -3′ |
| 5′-CCATAGCCATACTTCCCTTGTC-3′ | |
| 36B4 control mRNA | 5′-GTGTTCGACAATGGCAGCAT-3′ |
| 5′-GACACCCTCCAGGAAGCGA-3′ |
For agarose gel analysis, MCF-7 cells were maintained as above and treated with ethanol vehicle or 10 nM E2 for 15, 45 or 120 min before chromatin was isolated as described above using antibodies specific to ERα, PCNA or non-specific mouse IgG (sc-8002, sc-56 and sc-2025, respectively, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Purified ChIP DNA or 10% of input was subjected to 30 cycles of PCR with 0.5–1.0 mM MgCl2 and iTaq DNA polymerase (BioRad, Hercules, CA, USA) in a 20 μl reaction according to manufacturer's directions using primers flanking the oxytocin ERE-containing gene region, or a control region of the non-estrogen-responsive 36B4 gene which lacks an ERα-binding sequence (Table 1). The entire PCR reaction was run on a 1.5% agarose gel and visualized using SYBR Safe DNA gel stain (Molecular Probes, Eugene, OR, USA) under UV light on a Gel Doc with Quantity One software (BioRad, Hercules, CA, USA).
RNA interference assays
For siRNA experiments, MCF-7 cells were maintained as stated above and seeded in 12 well plates 24 h prior to transfection. Cells were transfected with 50 pmol of control (renilla luciferase, 4630, Ambion, Austin, TX, USA) or PCNA-specific siRNA oligos (Silencer validated siRNA ID 42853, Ambion, Austin, TX, USA) in the absence of antibiotics using siLentFect (BioRad, Hercules, CA, USA) for 48 h. Medium was replaced with phenol red-free MEM containing 5% v/v CDCS for an additional 24 h, followed by treatment with 10 nM E2 or ethanol vehicle for 24 h. Preliminary time course experiments were performed with 0–72 h of siRNA exposure to determine the amount of time required to maintain reduced PCNA protein levels. Protein knockdown was monitored by western blot analysis of whole cell lysates as described above using antibodies to PCNA and Sp1 (sc-56 and sc-59, respectively, Santa Cruz Biotechnologies, Santa Cruz, CA, USA). RNA was harvested using Trizol (Invitrogen, Carlsbad, CA, USA) and processed according to manufacturer's directions. cDNA was synthesized using the Reverse Transcription System (Promega, Madison, WI, USA). Real-time PCR was performed using iQ SYBR Green Supermix and the iCycler PCR thermocycler (BioRad, Hercules, CA, USA) according to manufacturer's directions with primer sequences specific for the PCNA, progesterone receptor (PR), pS2 and ERα mRNA (Table 1). The 36B4 gene, which is not regulated by E2, was used as a control. Samples were run in duplicate for each primer set during each experiment and standard curves were derived using serial dilutions of cDNA equivalent to 0.02, 0.2, 2 and 20 ng of input RNA. The relative nanogram of RNA was determined from the standard curve. Significant changes in RNA levels due to specific siRNA or hormone exposure were calculated by analysis of variance (ANOVA) with ezANOVA (C. Rorden, www.mricro.com, Columbia, SC, USA).
RESULTS
Isolation and identification of PCNA as a potential ERα-interacting protein
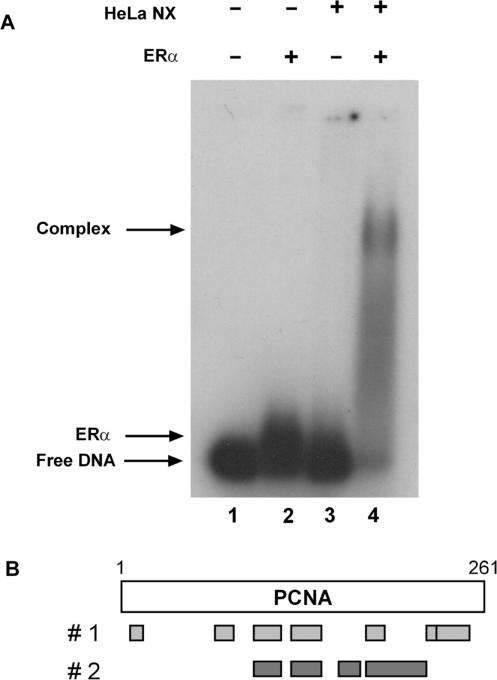
To isolate novel proteins associated with the DNA-bound ERα, we performed agarose-based gel mobility shift assays with 32P-labeled ERE-containing oligos, purified ERα and HeLa nuclear extracts. Although ERα slightly altered the migration of the ERE-containing oligos (Figure 1A, compare lanes 1 and 2), HeLa nuclear extracts alone did not alter migration of the radiolabeled probe (compare lanes 1 and 3). However, when both ERα and HeLa nuclear extracts were utilized, a higher order protein–DNA complex was formed (lane 4). We have shown previously that these higher-order complexes are supershifted by an ERα-specific antibody (23). This large protein–DNA complex was excised from the gel, proteins were subjected to trypsin digestion and the peptides were subjected to mass spectrometry analysis. Nine discrete peptides, which had amino acid sequence identical to that found in PCNA, were identified. As seen in Figure 1B, these peptides, which were identified in two independent experiments, represented a major portion (57%) of the PCNA protein. Since we had previously identified two DNA repair proteins, N-methylpurine DNA glycosylase (MPG) and FEN-1, which alter the ability of ERα to activate gene expression (21,22), we were intrigued by the identification of another DNA repair protein associated with the DNA-bound receptor.
Figure 1.
Isolation of PCNA. (A) Radiolabeled ERE-containing oligos were incubated with purified ERα in the absence or presence of HeLa nuclear extracts as indicated. Protein–DNA complexes were resolved on an agarose gel, excised and analyzed by mass spectrometry. (B) The locations of peptides identified by mass spectrometry analysis in two independent experiments are indicated by gray bars.
PCNA and ERα interact
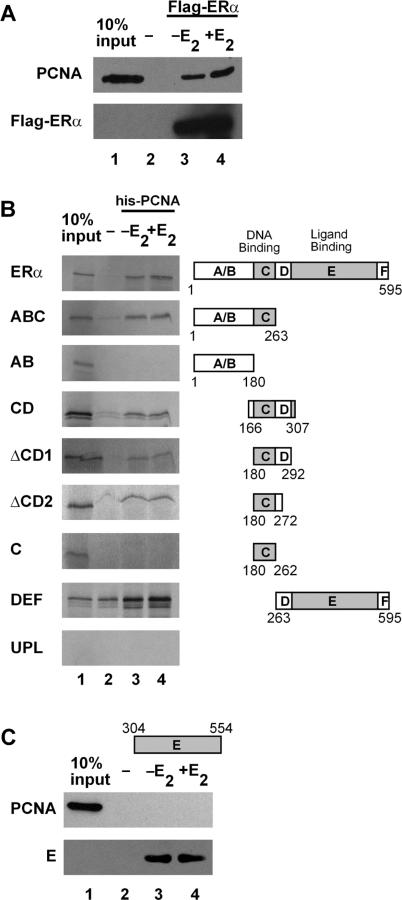
Because PCNA was isolated as a component of a large, multi-protein complex associated with the DNA-bound ERα, it was possible that other proteins were required for the ERα–PCNA interaction. To determine whether purified PCNA and ERα could interact, flag-tagged ERα was immobilized and incubated with purified, untagged PCNA. The purified PCNA was able to interact with the immobilized ERα in the absence and in the presence of E2 (Figure 2A, lanes 3 and 4), but did not interact with the resin alone (lane 2). Thus, PCNA interacts directly with ERα.
Figure 2.
PCNA interacts with ERα. (A) Flag affinity resin without (lanes 2) or with (lanes 3 and 4) purified, flag-tagged ERα was combined with purified, untagged PCNA. (B) Nickel affinity resin without (lanes 2) or with (lanes 3 and 4) purified PCNA was combined with in vitro translated 35S-labeled full-length or truncated ERα or unprogrammed lysate (UPL). (C) Nickel affinity resin without (lanes 2) or with (lanes 3 and 4) purified his-tagged ERα E domain was combined with purified, untagged PCNA. E2 was added as indicated. Proteins were separated on a denaturing gel and proteins were detected by western analysis (A and C) or autoradiography (B). Ten percent of PCNA input was included for reference (lane 1). Results are representative of three independent experiments.
To define which regions of the receptor are required for interaction with PCNA, bacterially expressed, his-tagged PCNA was immobilized and incubated with in vitro transcribed and translated full-length or truncated ERα. As shown in Figure 2B, full-length ERα and the amino-terminal ABC interacted with PCNA in the absence and in the presence of E2. Deletion of the C domain abolished the interaction with PCNA (AB). The failure of AB alone to interact with PCNA may reflect the relatively unstructured nature of the AB domains in the absence of the C domain (41–43).
Interestingly, a small portion of ERα containing only the DBD and hinge region interacted strongly with PCNA (CD). To further identify the central region of ERα required for PCNA interaction, sequential truncations of the hinge region were made. When 15 (▵CD1) or 35 (▵CD2) amino acids of the D domain were deleted, the interaction of ERα with PCNA was maintained. However, deletion of the entire D domain to amino acid 262 (C) eliminated the interaction of the two proteins. Thus, the inclusion of amino acids 262–272, which were previously defined as the C-terminal extension (CTE) of the DBD (44,45), is required for interaction with PCNA. Interestingly, the CTE not only stabilizes the receptor–DNA interaction, but also serves as a site for ERα acetylation, which in turn influences ERα-mediated transcription (46–48).
In addition to interacting with the central portion of ERα, PCNA also interacted with the DEF domains. Unfortunately, because the ERα LBD (E) interacted with the resin used for PCNA immobilization (data not shown), we were unable to determine whether PCNA interacted with the E domain using this method. However, his-tagged E domain was expressed, immobilized on nickel-NTA resin and incubated with untagged, purified PCNA. The his-tagged E domain bound to the resin (Figure 2C, lower panel), but unlike the full-length ERα (Figure 2A, upper panel), failed to interact with PCNA (Figure 2C, upper panel) suggesting that additional receptor domains must be required for PCNA interaction. Thus, while neither AB, C, nor E alone was able to interact with PCNA, combining these individual domains with additional, adjacent amino acid sequence induced formation of specific structural features required for PCNA interaction. These findings are in agreement with previous studies carried out with the progesterone and glucocorticoid receptors, which highlighted the interdependence of the receptor domains in maintaining the structural and functional integrity of these proteins (41–43).
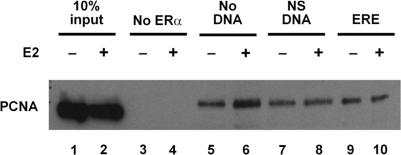
Since DNA can induce allosteric changes in ERα conformation (49–52), it seemed possible that binding of the receptor to DNA might influence the ERα–PCNA interaction. Baculovirus-expressed, flag-tagged ERα was immobilized on anti-flag resin and incubated with nuclear extracts from MCF-7 breast cancer cells, which express endogenous PCNA (Figure 3, lanes 1 and 2). ERα and its associated proteins were isolated, run on a denaturing polyacrylamide gel, and subjected to western blot analysis. PCNA interacted with ERα (lanes 5 and 6), but not with the resin alone (lanes 3 and 4). The interaction of PCNA with ERα was not influenced by addition of oligos containing a non-specific DNA sequence (lanes 7 and 8) or an ERE (lanes 9 and 10). Thus, in contrast to another DNA repair protein, MPG, which interacts more efficiently with the ERE-bound receptor than with free ERα (21), PCNA interacted with ERα in the absence and in the presence of DNA.
Figure 3.
ERα interacts with endogenously expressed PCNA. Flag-affinity resin without (lanes 3 and 4) or with flag-tagged ERα (lanes 5–10) was combined with 20 μg MCF-7 nuclear extract in the absence (lanes 3–6) or presence of oligos containing a non-specific DNA sequence (lanes 7 and 8) or an ERE (lanes 9 and 10). E2 was added as indicated. ERα and its associated proteins were eluted and separated on a denaturing gel. PCNA was detected by western analysis. Ten percent input was included for reference (lanes 1 and 2). Results are representative of two independent experiments.
PCNA enhances the ERα–ERE interaction
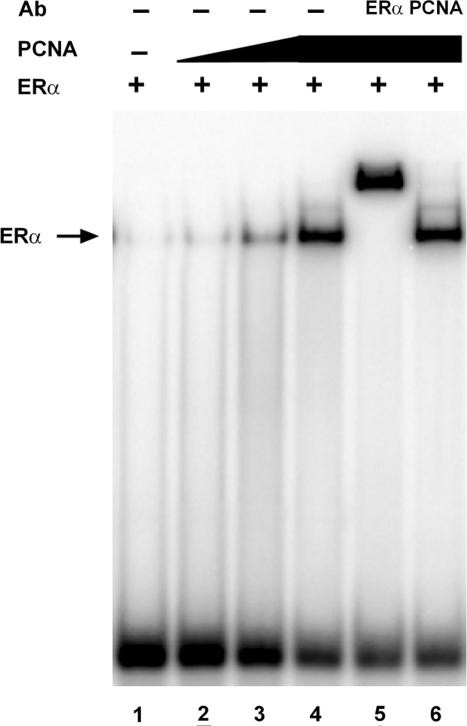
Although the addition of DNA did not influence the ERα–PCNA interaction, it was possible that the interaction of PCNA with ERα might alter the ability of the receptor to bind efficiently to DNA. To determine whether PCNA influenced ERα–ERE complex formation, gel mobility shift assays were performed with purified ERα in the absence and in the presence of increasing amounts of purified, his-tagged PCNA. When 10 fmol of purified ERα was combined with radiolabeled ERE-containing oligos, the receptor–DNA complex was barely detectable (Figure 4, lane 1). However, a dose-dependent increase in complex formation was seen when increasing amounts of PCNA were included in the binding reactions (lanes 2–4). Inclusion of an ERα-specific antibody (lane 5), but not a PCNA-specific antibody (lane 6), supershifted the receptor–DNA complex. Thus, although PCNA dramatically increased the ERα–ERE interaction, it was not present in the receptor–DNA complex. The failure of PCNA to form a stable ternary complex with the DNA-bound ERα could result from the absence of other nuclear proteins required for stabilization of the PCNA–receptor–DNA interaction and/or the extended period of electrophoresis required for gel mobility shift assays, which could prohibit the formation of a stable ternary complex containing these two purified proteins. The ability of purified co-regulatory proteins to enhance the receptor–DNA complex, but not form a stable ternary complex in gel mobility shift assays, has been reported with a number of ERα-associated proteins by our laboratory and others (23,53–57), including the DNA repair proteins MPG and FEN-1 (21,22). Furthermore, these findings are consistent with the ability of PCNA to enhance the interaction of FEN-1 with DNA but not form a ternary complex with the DNA-bound FEN-1 (58).
Figure 4.
PCNA enhances ERα–ERE complex formation. 32P-labeled oligos containing the consensus ERE were incubated with 10 fmol of purified ERα in the presence of E2.Purified his-labeled PCNA (lanes 2–6) and ERα- (lane 5) or PCNA- (lane 6) specific antibody (Ab) were added to the binding reactions as indicated. Bound and unbound 32P-labeled oligos were fractionated on a non-denaturing polyacrylamide gel and visualized by autoradiography. Complexes containing ERα are indicated (ERα→). Results are representative of three independent experiments.
Association of PCNA with estrogen-responsive genes
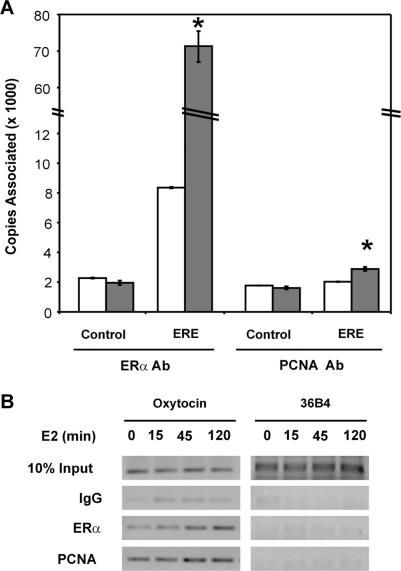
Although we were unable to isolate a ternary complex containing purified ERα and PCNA with DNA in our gel mobility shift experiments, it seemed possible that PCNA might be able to interact with an ERE-containing region of an endogenous estrogen-responsive gene in its native chromatin environment. Thus, chromatin immunoprecipitation assays were performed to examine the interaction of endogenously expressed ERα and PCNA with the native pS2 gene in MCF-7 cells using real-time PCR analysis. Consistent with previous studies (22,23,59,60), more ERα was associated with the ERE-containing region of the pS2 gene in the presence than in the absence of E2 (Figure 5A). In contrast, no change was observed in the association of ERα with a region 2.8-kb upstream of the pS2 ERE (Control), which lacked an ERα-binding site. Likewise, when a PCNA-specific antibody was utilized, there was no change in the association of PCNA with the upstream pS2 region in the absence or presence of E2. However, a modest, statistically significant increase was observed in the association of PCNA with the ERE-containing region of the pS2 gene in the presence of E2.
Figure 5.
PCNA associates with endogenous estrogen-responsive genes. (A) Sheared chromatin from MCF-7 cells, which had been treated with ethanol vehicle (white bars) or 10 nM E2 for 2 h (gray bars), was immunoprecipitated with an ERα- or PCNA-specific antibody. DNA was isolated and real-time PCR was performed in triplicate to monitor the association of ERα and PCNA with the region of the pS2 gene containing an imperfect ERE or a region 2.8-kb upstream of the pS2 ERE (Control). Standard curves were derived for each primer set and the relative copy number for each sample was obtained based on the standard curve. Data from four independent experiments are expressed as the mean ± SEM. A significant change in the copies associated induction in the presence of E2 was determined by Student's t-test and is indicated by an asterisk (*, P < 0.05). (B) Sheared chromatin from MCF-7 cells, which had been treated with ethanol vehicle or 10 nM E2 for 15, 45 or 120 min, was immunoprecipitated with an ERα- or PCNA-specific antibody or non-specific IgG. DNA was isolated and subjected to PCR amplification to monitor the association of ERα and PCNA with the ERE-containing region of the oxytocin gene or the non-estrogen-responsive 36B4 gene. Ten percent of input DNA was included as a control. PCR products were run on 1.5% agarose gels, stained and visualized using UV light. Results are representative of three independent experiments.
We also examined another ERE-containing gene region using agarose gels to visualize the PCR products. While both ERα and PCNA were present at the ERE-containing region of the oxytocin gene in the absence of hormone, more ERα and PCNA were associated with this gene region when cells had been treated with E2 for 45 min or 2 h. The protection of the pS2 ERE and the association of ERα with the ERE-containing region of the pS2 gene in the absence of hormone have been reported previously (38,61). In contrast, neither ERα nor PCNA was associated with the 36B4 gene, which contains no ERα-binding site and is unaffected by hormone treatment. When a non-specific IgG control antibody was utilized, the amount of amplicon produced was far less than observed when an ERα- or PCNA-specific antibody was used. Thus, PCNA was present at the ERE-containing region of the estrogen-responsive oxytocin gene in the absence and in the presence of hormone. Combined with our gel shift assays, which demonstrated that PCNA enhances the ERα–ERE interaction, these findings suggest that PCNA may help to stabilize the interaction of ERα with endogenous, ERE-containing DNA regions in the absence and in the presence of E2.
Effect of PCNA on transcription
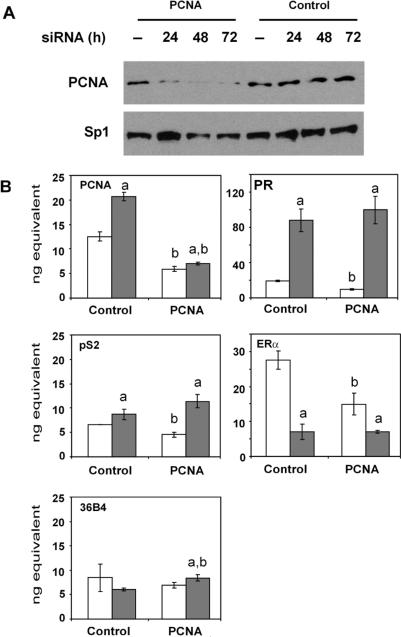
The ability of PCNA to enhance the ERα–ERE interaction and associate with estrogen-responsive genes suggested that it might be able to influence ERα-mediated transcription. To examine the potential effect of PCNA on transcription of endogenous, estrogen-responsive genes in their native chromatin environment, siRNA was employed to knock down PCNA expression in MCF-7 cells. In addition, siRNA directed against renilla luciferase was used as a control. When PCNA-specific siRNA was used, PCNA protein levels were decreased and remained low for 72 h after siRNA treatment (Figure 6A). In contrast, PCNA levels were not affected by control siRNA. The level of Sp1 protein was also monitored to help ensure that neither of the siRNAs utilized had an overall effect on protein expression. Sp1 is a transcription factor that plays an important role in regulating expression of a number of estrogen-responsive genes (62) including the progesterone receptor (PR) gene (63–68). The level of PCNA mRNA was also examined using quantitative real-time PCR. Exposure of MCF-7 cells to E2 increased PCNA mRNA levels when control siRNA was used (Figure 6B). When PCNA-specific siRNA was used, significant decreases in PCNA mRNA were observed in the absence and in the presence of E2.
Figure 6.
Knocking down endogenous PCNA expression alters gene expression. MCF-7 cells were transfected with 50 pmol of double-stranded control siRNA directed against renilla luciferase (Control) or PCNA-specific siRNA. (A) After 0–72 h, whole cell lysates were subjected to western analysis using a PCNA- or Sp1-specific antibody. Results are representative of two independent experiments. (B) After 72 h, cells were treated with ethanol vehicle (white bars) or 10 nM E2 (gray bars) for 24 h, RNA was harvested and cDNA was synthesized. Real-time PCR was performed using primers specific to PCNA, PR, pS2, ERα or 36B4 mRNA sequences. Standard curves were derived for each primer set in each experiment. The relative amount of RNA obtained for each sample was calculated from the standard curve. Data are reported as the mean of three replicates ± SEM. One representative of four independent experiments is shown. Significant differences (P < 0.05) in E2-treated cells compared to the corresponding ethanol vehicle (a) or control siRNA (b) were determined by ANOVA and are indicated. Some error bars are too small to be visible.
To determine whether decreasing endogenous PCNA expression would alter the expression of endogenous estrogen-responsive genes, we examined the expression of the well-studied pS2 gene. Exposure of MCF-7 cells to E2 increased pS2 mRNA in the presence of control siRNA as has been reported previously (22,23). Interestingly, the PCNA-specific siRNA decreased basal pS2 mRNA levels, but did not affect estrogen-induced pS2 mRNA levels. We also examined expression of the human PR gene, which lacks a palindromic ERE sequence, but instead derives at least part of its estrogen responsiveness from multiple AP-1 and Sp1 sites (63–68). In agreement with previous studies (22,23), PR mRNA levels increased when MCF-7 cells were treated with E2 and control siRNA. Interestingly, as we had observed with the pS2 gene, basal PR mRNA levels were significantly decreased in the presence of PCNA-specific siRNA, but no change was observed in the level of PR mRNA when MCF-7 cells were treated with hormone.
Since ERα regulates estrogen responsiveness, we also examined the ERα mRNA levels. When control siRNA was used, an E2-induced decrease in ERα mRNA level was observed as has been reported previously (65,69). However, when PCNA-specific siRNA was utilized, ERα mRNA levels were decreased in the absence, but not in the presence of E2 as we had observed with the pS2 and PR genes. In contrast, no change was observed in the level of 36B4 mRNA, which is constitutively expressed, in the absence or in the presence of hormone when control siRNA was used. A slight increase in the level of 36B4 mRNA was detected in the presence of E2 when the PCNA-specific siRNA was used, but the magnitude of this increase was less than the decreases in basal mRNA levels observed for the estrogen-responsive genes.
These experiments demonstrate that although decreased PCNA levels did not alter the estrogen responsiveness of the PR, pS2 and ERα genes, significant decreases in basal mRNA levels were observed. Combined with our ChIP experiments, our findings suggest that PCNA may help to maintain basal expression of estrogen-responsive genes by stabilizing the interaction of the receptor with DNA in the absence of hormone.
DISCUSSION
We have identified a novel interaction between ERα and PCNA, a protein required for DNA replication and repair. We have shown that PCNA interacts with ERα, enhances the ERα–ERE interaction and helps to maintain basal expression of estrogen-responsive genes.
PCNA is present in cells as a homotrimer comprised of three PCNA monomers that encircle the DNA helix in a head-to-tail orientation (70). While associated with DNA, PCNA serves as a loading dock for proteins involved in DNA replication and repair. It binds to DNA polymerase δ and FEN-1 and increases their catalytic activities thereby enhancing DNA replication and processing of Okazaki fragments (58,71).PCNA participates in numerous DNA repair pathways including nucleotide excision repair, base excision repair, mismatch repair and double-strand break repair (72–77). The extraordinary versatility of PCNA is evident in its ability to interact with more than 20 polymerases, ligases, endonucleases and helicases involved in DNA replication and repair (78).
PCNA functions as a sliding clamp that advances along template DNA while recruiting DNA replication and repair factors and tethering them to DNA (79). Although the association of many replication factors with DNA is transient, the interaction of PCNA with DNA is more sustained (80). It has been suggested that the continued association of PCNA with DNA and the transient association of its binding partners enables PCNA to simultaneously coordinate DNA replication, DNA repair and cell-cycle progression (74,78). This persistent association of PCNA with DNA might explain the presence of PCNA at the endogenous ERE-containing gene regions in the absence of E2 and the modest increases we observed in the presence of E2.
In addition to its interaction with ERα, previous studies have documented the interaction of PCNA with other transcription factors including the retinoic acid receptor (RAR) and the nuclear receptor coactivator p300 (81,82). Since the E2-occupied ERα interacts with p300 (18,47,83,84) and p300 interacts with PCNA, PCNA may help to form an interconnected network of regulatory proteins associated with nuclear receptors to modulate gene expression in the presence of hormone. Our ChIP assays (Figure 5) suggest that PCNA may also be essential in stabilizing the receptor–DNA interaction and maintaining basal expression of estrogen-responsive genes in the absence of hormone, when fewer co-regulatory proteins are present to stabilize the ERα–ERE interaction. Thus, in addition to serving as a platform for the recruitment of proteins involved in DNA replication and repair, PCNA may serve as a platform for nuclear receptors and co-regulatory proteins involved in modulating transcription.
Recently, Ivanov et al. (85) used computer modeling to examine PCNA–DNA interaction and showed that PCNA contacts the minor groove of the DNA helix and causes the DNA to tilt. Previous work from our laboratory demonstrated that ERα CTE induces conformational changes in DNA structure resulting in compression of the major groove and expansion of the minor groove (39,86). Since the ERα CTE is required for interaction with PCNA and stabilizes the ERα–ERE interaction (46), it is possible that PCNA may foster the ERα–ERE interaction by altering DNA structure.
Although increased PCNA expression has been reported in proliferating normal cells (73,74,87–89) and at the site of uterine implantation (90,91), increased expression of PCNA has also been linked to decreased survival of breast cancer patients (29,92–94). Interestingly, a post-translational modification of PCNA that lowers its DNA replication fidelity has been found in MCF-7 cells and in malignant breast and ovarian cancers, but not in normal tissues (95,96). It has been suggested that this decreased fidelity of the modified PCNA in cancer cells may play a role in tumor progression and decreased survival, whereas the unmodified PCNA in normal cells may help to protect the integrity of the genome.
While PCNA is required for DNA replication and repair and has been used as a marker of proliferation, our studies expand the functional repertoire of PCNA from the realm of DNA replication and repair and highlight its involvement in altering gene expression.
ACKNOWLEDGEMENTS
We thank W.L. Kraus and J. Kadonaga for providing ERα viral stock, B. Katzenellenbogen, Z. Kelman and K. Nettles for expression plasmids, and J. Bruning and K. Nettles for purified PCNA. This work was supported by NIH grant R01 DK 53884 (to A.M.N.) and NIH P41 RR11823-10 (to J.R.Y.). J.R.S.-N. was supported by the NIH Reproductive Biology Training Grant (T32 HD07028). Funding to pay the Open Access publication charges for this article was provided by R01 DK 53884.
Conflict of interest statement. None declared.
REFERENCES
- 1.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 2.Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 2004;64:1522–1533. doi: 10.1158/0008-5472.can-03-3326. [DOI] [PubMed] [Google Scholar]
- 3.Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science. 1994;266:1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- 4.Hess RA, Bunick D, Lee K-H, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 6.Glass CK. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr. Rev. 1994;15:391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Green S, Stack G, Berry M, Jin J-R, Chambon P. Functional domains of the human estrogen receptor. Cell. 1987;51:941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- 8.Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 9.Metzger D, Ali S, Bornert J-M, Chambon P. Characterization of the amino-terminal transcriptional activation function of the human estrogen receptor in animal and yeast cells. J. Biol. Chem. 1995;270:9535–9542. doi: 10.1074/jbc.270.16.9535. [DOI] [PubMed] [Google Scholar]
- 10.Danielian PS, White R, Lees JA, Parker MG. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992;11:1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robyr D, Wolffe AP, Wahli W. Nuclear hormone receptor coregulators in action: diversity for shared tasks. Mol. Endocrinol. 2000;14:329–347. doi: 10.1210/mend.14.3.0411. [DOI] [PubMed] [Google Scholar]
- 12.Brou C, Wu J, Ali S, Scheer E, Lang C, Davidson I, Chambon P, Tora L. Different TBP-associated factors are required for mediating the stimulation of transcription in vitro by the acidic transactivator GAL-VP16 and the two nonacidic activation functions of the estrogen receptor. Nucleic Acids Res. 1993;21:5–12. doi: 10.1093/nar/21.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oñate SA, Tsai SY, Tsai M-J, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 14.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan X-Y, Sauter G, Kallioniemi O-P, Trent JM, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 16.Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transciptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol. Cell. Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc. Natl Acad. Sci. USA. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakravarti D, LaMorte VJ, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM. Role of CBP/P300 in nuclear receptor signaling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 19.Hanstein B, Eckner R, DiRenzo J, Halachmi S, Liu H, Searcy B, Kurokawa R, Brown M. p300 is a component of an estrogen receptor coactivator complex. Proc. Natl Acad. Sci. USA. 1996;93:11540–11545. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L. Nuclear receptor coactivators and corepressors. Mol. Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 21.Likhite VS, Cass EI, Anderson SD, Yates JR, Nardulli AM. Interaction of estrogen receptor alpha with 3-methyladenine DNA glycosylase modulates transcription and DNA repair. J. Biol. Chem. 2004;279:16875–16882. doi: 10.1074/jbc.M313155200. [DOI] [PubMed] [Google Scholar]
- 22.Schultz-Norton JR, Walt KA, Ziegler YS, McLeod IX, Yates JR, Raetzman LT, Nardulli AM. The deoxyribonucleic acid repair protein flap endonuclease-1 modulates estrogen-responsive gene expression. Mol. Endocrinol. 2007;21:1569–1580. doi: 10.1210/me.2006-0519. [DOI] [PubMed] [Google Scholar]
- 23.Schultz-Norton JR, McDonald WH, Yates JR, Nardulli AM. Protein disulfide isomerase serves as a molecular chaperone to maintain estrogen receptor {alpha} structure and function. Mol. Endocrinol. 2006;20:1982–1995. doi: 10.1210/me.2006-0006. [DOI] [PubMed] [Google Scholar]
- 24.Hosfield DJ, Mol CD, Shen B, Tainer JA. Structure of the DNA repair and replication endonuclease and exonuclease FEN-1: coupling DNA and PCNA binding to FEN-1 activity. Cell. 1998;95:135–146. doi: 10.1016/s0092-8674(00)81789-4. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai S, Kitano K, Yamaguchi H, Hamada K, Okada K, Fukuda K, Uchida M, Ohtsuka E, Morioka H, et al. Structural basis for recruitment of human flap endonuclease 1 to PCNA. EMBO J. 2005;24:683–693. doi: 10.1038/sj.emboj.7600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakurai S, Kitano K, Okada K, Hamada K, Morioka H, Hakoshima T. Preparation and crystallization of human flap endonuclease FEN-1 in complex with proliferating-cell nuclear antigen, PCNA. Acta Crystallogr. Sect. D Biol. Crystallogr. 2003;59:933–935. doi: 10.1107/s0907444903004815. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji T, Kitajima S, Koashi Y. Expression of proliferating cell nuclear antigen (PCNA) and apoptosis related antigen (LeY) in epithelial skin tumors. Am. J. Dermatopathol. 1998;20:164–169. doi: 10.1097/00000372-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Horiguchi J, Iino Y, Takei H, Maemura M, Takeyoshi I, Yokoe T, Ohwada S, Oyama T, Nakajima T, et al. Long-term prognostic value of PCNA labeling index in primary operable breast cancer. Oncol. Rep. 1998;5:641–644. doi: 10.3892/or.5.3.641. [DOI] [PubMed] [Google Scholar]
- 29.Chu JS, Huang CS, Chang KJ. Proliferating cell nuclear antigen (PCNA) immunolabeling as a prognostic factor in invasive ductal carcinoma of the breast in Taiwan. Cancer Lett. 1998;131:145–152. doi: 10.1016/s0304-3835(98)00118-9. [DOI] [PubMed] [Google Scholar]
- 30.Kong C, Li Z, Liu T. Expression and clinical significance of PCNA in renal pelvic and ureteral cancer. Zhonghua Wai Ke Za Zhi. 1996;34:614–616. [PubMed] [Google Scholar]
- 31.Kelman Z, Yao N, O’Donnell M. Escherichia coli expression vectors containing a protein kinase recognition motif, His6-tag and hemagglutinin epitope. Gene. 1995;166:177–178. doi: 10.1016/0378-1119(95)00556-7. [DOI] [PubMed] [Google Scholar]
- 32.Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc. Natl Acad. Sci. USA. 1999;96:6947–6952. doi: 10.1073/pnas.96.12.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delage-Mourroux R, Martini PG, Choi I, Kraichely DM, Hoeksema J, Katzenellenbogen BS. Analysis of estrogen receptor interaction with a repressor of estrogen receptor activity (REA) and the regulation of estrogen receptor transcriptional activity by REA. J. Biol. Chem. 2000;275:35848–35856. doi: 10.1074/jbc.M001327200. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Klooster S, Shapiro DJ. Intrinsically bent DNA in a eukaryotic transcription factor recognition sequence potentiates transcription activation. J. Biol. Chem. 1995;270:1282–1288. doi: 10.1074/jbc.270.3.1282. [DOI] [PubMed] [Google Scholar]
- 35.Stols L, Gu M, Dieckman L, Raffen R, Collart FR, Donnelly MI. A new vector for high-throughput, ligation-independent cloning encoding a tobacco etch virus protease cleavage site. Protein Expr. Purif. 2002;25:8–15. doi: 10.1006/prep.2001.1603. [DOI] [PubMed] [Google Scholar]
- 36.Carlson KE, Choi I, Gee A, Katzenellenbogen BS, Katzenellenbogen JA. Altered ligand binding properties and enhanced stability of a constitutively active estrogen receptor: evidence that an open pocket conformation is required for ligand interaction. Biochemistry. 1997;36:14897–14905. doi: 10.1021/bi971746l. [DOI] [PubMed] [Google Scholar]
- 37.Loven MA, Wood JA, Nardulli AM. Interaction of estrogen receptors alpha and beta with estrogen response elements. Mol. Cell. Endocrinol. 2001;181:151–163. doi: 10.1016/s0303-7207(01)00491-9. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Petz LN, Ziegler YS, Wood JR, Potthoff SJ, Nardulli AM. Regulation of the estrogen-responsive pS2 gene in MCF-7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2000;74:157–168. doi: 10.1016/s0960-0760(00)00119-9. [DOI] [PubMed] [Google Scholar]
- 39.Schultz JR, Loven MA, Senkus Melvin VM, Edwards DP, Nardulli AM. Differential modulation of DNA conformation by estrogen receptors {alpha} and {beta} J. Biol. Chem. 2002;277:8702–8707. doi: 10.1074/jbc.M108491200. [DOI] [PubMed] [Google Scholar]
- 40.Chodosh LA, Buratowski S. In: Current Protocols in Molecular Biology. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Vol. 2. New York: Greene Publishing Associates and Wiley Interscience; 1989. pp. 12.2.1–12.2.10. [Google Scholar]
- 41.Kumar R, Baskakov IV, Srinivasan G, Bolen DW, Lee JC, Thompson EB. Interdomain signaling in a two-domain fragment of the human glucocorticoid receptor. J. Biol. Chem. 1999;274:24737–24741. doi: 10.1074/jbc.274.35.24737. [DOI] [PubMed] [Google Scholar]
- 42.Bain DL, Franden MA, McManaman JL, Takimoto GS, Horwitz KB. The N-terminal region of the human progesterone A-receptor. J. Biol. Chem. 2000;275:7313–7320. doi: 10.1074/jbc.275.10.7313. [DOI] [PubMed] [Google Scholar]
- 43.Bain DL, Franden MA, McManaman JL, Takimoto GS, Horwitz KB. The N-terminal region of human progesterone B-receptors: biophysical and biochemical comparison to A-receptors. J. Biol. Chem. 2001;276:23825–23831. doi: 10.1074/jbc.M102611200. [DOI] [PubMed] [Google Scholar]
- 44.Melvin VS, Harrell C, Adelman JS, Kraus WL, Churchill M, Edwards DP. The role of the C-terminal extension (CTE) of the estrogen receptor alpha and beta DNA binding domain in DNA binding and interaction with HMGB. J. Biol. Chem. 2004;279:14763–14771. doi: 10.1074/jbc.M313335200. [DOI] [PubMed] [Google Scholar]
- 45.Melvin VS, Roemer SC, Churchill ME, Edwards DP. The C-terminal extension (CTE) of the nuclear hormone receptor DNA binding domain determines interactions and functional response to the HMGB-1/-2 co-regulatory proteins. J. Biol. Chem. 2002;277:25115–25124. doi: 10.1074/jbc.M110400200. [DOI] [PubMed] [Google Scholar]
- 46.Mader S, Chambon P, White JH. Defining a minimal estrogen receptor DNA binding domain. Nucleic Acids Res. 1993;21:1125–1132. doi: 10.1093/nar/21.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol. Endocrinol. 2006;20:1479–1493. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roemer SC, Donham DC, Sherman L, Pon VH, Edwards DP, Churchill ME. Structure of the progesterone receptor-deoxyribonucleic acid complex: novel interactions required for binding to half-site response elements. Mol. Endocrinol. 2006;20:3042–3052. doi: 10.1210/me.2005-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood JR, Greene GL, Nardulli AM. Estrogen response elements function as allosteric modulators of estrogen receptor conformation. Mol. Cell. Biol. 1998;18:1927–1934. doi: 10.1128/mcb.18.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood JR, Likhite VS, Loven MA, Nardulli AM. Allosteric modulation of estrogen receptor conformation by different estrogen response elements. Mol. Endocrinol. 2001;15:1114–1126. doi: 10.1210/mend.15.7.0671. [DOI] [PubMed] [Google Scholar]
- 51.Hall JM, McDonnell DP, Korach KS. Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements. Mol. Endocrinol. 2002;16:469–486. doi: 10.1210/mend.16.3.0814. [DOI] [PubMed] [Google Scholar]
- 52.Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J. Mol. Endocrinol. 2004;33:387–410. doi: 10.1677/jme.1.01541. [DOI] [PubMed] [Google Scholar]
- 53.Landel CC, Kushner PJ, Greene GL. The interaction of human estrogen receptor with DNA is modulated by receptor-associated proteins. Mol. Endocrinol. 1994;8:1407–1419. doi: 10.1210/mend.8.10.7854357. [DOI] [PubMed] [Google Scholar]
- 54.Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi ME, Taraseviciene L, Nordeen SK, Allegretto EA, et al. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol. Cell. Biol. 1998;18:4471–4487. doi: 10.1128/mcb.18.8.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romine L, Wood J, Lamia L, Prendergast P, Edwards D, Nardulli A. The high mobility group protein 1 enhances binding of the estrogen receptor DNA binding domain to the estrogen response element. Mol. Endocrinol. 1998;12:664–674. doi: 10.1210/mend.12.5.0111. [DOI] [PubMed] [Google Scholar]
- 56.Loven MA, Muster N, Yates JR, Nardulli AM. A novel estrogen receptor alpha associated protein, template activating factor I beta, inhibits acetylation and transactivation. Mol. Endocrinol. 2003;17:67–78. doi: 10.1210/me.2002-0280. [DOI] [PubMed] [Google Scholar]
- 57.Loven MA, Davis RE, Curtis CD, Muster N, Yates JR, Nardulli AM. A novel estrogen receptor alpha-associated protein alters receptor-deoxyribonucleic acid interactions and represses receptor-mediated transcription. Mol. Endocrinol. 2004;18:2649–2659. doi: 10.1210/me.2003-0195. [DOI] [PubMed] [Google Scholar]
- 58.Tom S, Henricksen LA, Bambara RA. Mechanism whereby proliferating cell nuclear antigen stimulates flap endonuclease 1. J. Biol. Chem. 2000;275:10498–10505. doi: 10.1074/jbc.275.14.10498. [DOI] [PubMed] [Google Scholar]
- 59.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 60.Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 61.Chen H, Lin RJ, Xie W, Wilpitz D, Evans RM. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell. 1999;98:675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 62.Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam. Horm. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]
- 63.Petz LN, Nardulli AM. Sp1 binding sites and an estrogen resonse element half-site are involved in regulation of the human progesterone receptor A promoter. Mol. Endocrinol. 2000;14:972–985. doi: 10.1210/mend.14.7.0493. [DOI] [PubMed] [Google Scholar]
- 64.Petz LN, Ziegler YS, Loven MA, Nardulli AM. Estrogen receptor alpha and activating protein-1 mediate estrogen responsiveness of the progesterone receptor gene in MCF-7 breast cancer cells. Endocrinology. 2002;143:4583–4591. doi: 10.1210/en.2002-220369. [DOI] [PubMed] [Google Scholar]
- 65.Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM. Differential regulation of the human progesterone receptor gene by an estrogen response element half site and Sp1 sites. J. Steroid Biochem. Mol. Biol. 2004;88:113–122. doi: 10.1016/j.jsbmb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Petz LN, Ziegler YS, Schultz JR, Nardulli AM. Fos and Jun inhibit estrogen-induced transcription of the human progesterone receptor gene through an activator protein-1 site. Mol. Endocrinol. 2004;18:521–532. doi: 10.1210/me.2003-0105. [DOI] [PubMed] [Google Scholar]
- 67.Schultz JR, Petz LN, Nardulli AM. Estrogen receptor α and Sp1 regulate progesterone receptor gene expression. Mol. Cell. Endocrinol. 2003;201:165–175. doi: 10.1016/s0303-7207(02)00415-x. [DOI] [PubMed] [Google Scholar]
- 68.Schultz JR, Petz LN, Nardulli AM. Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors α and β. J. Biol. Chem. 2005;280:347–354. doi: 10.1074/jbc.M407879200. [DOI] [PubMed] [Google Scholar]
- 69.Monsma FJ, Katzenellenbogen B, Miller M, Ziegler Y, Ja K. Characterization of the estrogen receptor and its dynamics in MCF-7 human breast cancer cells using a covalently attaching antiestrogen. Endocrinology. 1984;115:143–153. doi: 10.1210/endo-115-1-143. [DOI] [PubMed] [Google Scholar]
- 70.Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell. 1994;79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 71.Maga G, Stucki M, Spadari S, Hubscher U. DNA polymerase switching: I. replication factor C displaces DNA polymerase alpha prior to PCNA loading. J. Mol. Biol. 2000;295:791–801. doi: 10.1006/jmbi.1999.3394. [DOI] [PubMed] [Google Scholar]
- 72.Shivji KK, Kenny MK, Wood RD. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992;69:367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- 73.Aboussekhra A, Wood RD. Detection of nucleotide excision repair incisions in human fibroblasts by immunostaining for PCNA. Exp. Cell Res. 1995;221:326–332. doi: 10.1006/excr.1995.1382. [DOI] [PubMed] [Google Scholar]
- 74.Li R, Hannon GJ, Beach D, Stillman B. Subcellular distribution of p21 and PCNA in normal and repair-deficient cells following DNA damage. Curr. Biol. 1996;6:189–199. doi: 10.1016/s0960-9822(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 75.Gary R, Kim K, Cornelius HL, Park MS, Matsumoto Y. Proliferating cell nuclear antigen facilitates excision in long-patch base excision repair. J. Biol. Chem. 1999;274:4354–4363. doi: 10.1074/jbc.274.7.4354. [DOI] [PubMed] [Google Scholar]
- 76.Lau PJ, Kolodner RD. Transfer of the MSH2.MSH6 complex from proliferating cell nuclear antigen to mispaired bases in DNA. J. Biol. Chem. 2003;278:14–17. doi: 10.1074/jbc.C200627200. [DOI] [PubMed] [Google Scholar]
- 77.Lebel M, Spillare EA, Harris CC, Leder P. The Werner syndrome gene product co-purifies with the DNA replication complex and interacts with PCNA and topoisomerase I. J. Biol. Chem. 1999;274:37795–37799. doi: 10.1074/jbc.274.53.37795. [DOI] [PubMed] [Google Scholar]
- 78.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J. Cell Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 79.Kelman Z, O’Donnell M. Structural and functional similarities of prokaryotic and eukaryotic DNA polymerase sliding clamps. Nucleic Acids Res. 1995;23:3613–3620. doi: 10.1093/nar/23.18.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sporbert A, Domaing P, Leonhardt H, Cardoso MC. PCNA acts as a stationary loading platform for transiently interacting Okazaki fragment maturation proteins. Nucleic Acids Res. 2005;33:3521–3528. doi: 10.1093/nar/gki665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin PJ, Lardeux V, Lefebvre P. The proliferating cell nuclear antigen regulates retinoic acid receptor transcriptional activity through direct protein-protein interaction. Nucleic Acids Res. 2005;33:4311–4321. doi: 10.1093/nar/gki745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong R, Chakravarti D. The human proliferating cell nuclear antigen regulates transcriptional coactivator p300 activity and promotes transcriptional repression. J. Biol. Chem. 2003;278:44505–44513. doi: 10.1074/jbc.M303138200. [DOI] [PubMed] [Google Scholar]
- 83.Kraus WL, Kadonaga JT. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev. 1998;12:331–342. doi: 10.1101/gad.12.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamstein B, Eckner R, DiRenzo J, Halachmi S, Liu H, Searcy B, Kurokawa R, Brown M. p300 is a component of an estrogen receptor coactivator complex. Proc. Natl Acad. Sci. USA. 1996;21:11540–11545. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ivanov I, Chapados BR, McCammon JA, Tainer JA. Proliferating cell nuclear antigen loaded onto double-stranded DNA: dynamics, minor groove interactions and functional implications. Nucleic Acids Res. 2006;34:6023–6033. doi: 10.1093/nar/gkl744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nardulli AM, Grobner C, Cotter D. Estrogen receptor-induced DNA bending: orientation of the bend and replacement of an estrogen response element with an intrinsic DNA bending sequence. Mol. Endocrinol. 1995;9:1064–1076. doi: 10.1210/mend.9.8.7476980. [DOI] [PubMed] [Google Scholar]
- 87.Kim IS, Lee MY, Lee IH, Shin SL, Lee SY. Gene expression of flap endonuclease-1 during cell proliferation and differentiation. Biochim. Biophys. Acta. 2000;1496:333–340. doi: 10.1016/s0167-4889(00)00029-x. [DOI] [PubMed] [Google Scholar]
- 88.Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J. Immunol. 1978;121:2228–2234. [PubMed] [Google Scholar]
- 89.Solovjeva L, Svetlova M, Sasina L, Tanaka K, Saijo M, Nazarov I, Bradbury M, Tomilin N. High mobility of flap endonuclease 1 and DNA polymerase eta associated with replication foci in mammalian S-phase nucleus. Mol. Biol. Cell. 2005;16:2518–2528. doi: 10.1091/mbc.E04-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Korgun ET, Celik-Ozenci C, Acar N, Cayli S, Desoye G, Demir R. Location of cell cycle regulators cyclin B1, cyclin A, PCNA, Ki67 and cell cycle inhibitors p21, p27 and p57 in human first trimester placenta and deciduas. Histochem. Cell. Biol. 2006;125:615–624. doi: 10.1007/s00418-006-0160-y. [DOI] [PubMed] [Google Scholar]
- 91.Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J. Biol. Chem. 2001;276:44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- 92.Siitonen SM, Kallioniemi OP, Isola JJ. Proliferating cell nuclear antigen immunohistochemistry using monoclonal antibody 19A2 and a new antigen retrieval technique has prognostic impact in archival paraffin-embedded node-negative breast cancer. Am. J. Pathol. 1993;142:1081–1089. [PMC free article] [PubMed] [Google Scholar]
- 93.Aaltomaa S, Lipponen P, Syrjanen K. Proliferating cell nuclear antigen (PCNA) immunolabeling as a prognostic factor in axillary lymph node negative breast cancer. Anticancer Res. 1993;13:533–538. [PubMed] [Google Scholar]
- 94.Aaltomaa S, Lipponen P, Papinaho S, Syrjanen K. Proliferating-cell nuclear antigen (PC10) immunolabelling and other proliferation indices as prognostic factors in breast cancer. J. Cancer Res. Clin. Oncol. 1993;119:288–294. doi: 10.1007/BF01212727. [DOI] [PubMed] [Google Scholar]
- 95.Malkas LH, Herbert BS, Abdel-Aziz W, Dobrolecki LE, Liu Y, Agarwal B, Hoelz D, Badve S, Schnaper L, et al. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. Proc. Natl Acad Sci. USA. 2006;103:19472–19477. doi: 10.1073/pnas.0604614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoelz DJ, Arnold RJ, Dobrolecki LE, Abdel-Aziz W, Loehrer AP, Novotny MV, Schnaper L, Hickey RJ, Malkas LH. The discovery of labile methyl esters on proliferating cell nuclear antigen by MS/MS. Proteomics. 2006;6:4808–4816. doi: 10.1002/pmic.200600142. [DOI] [PubMed] [Google Scholar]