Abstract
Chlorophyll (Chl) synthase catalyzes esterification of chlorophyllide to complete the last step of Chl biosynthesis. Although the Chl synthases and the corresponding genes from various organisms have been well characterized, Chl synthase mutants have not yet been reported in higher plants. In this study, a rice (Oryza Sativa) Chl-deficient mutant, yellow-green leaf1 (ygl1), was isolated, which showed yellow-green leaves in young plants with decreased Chl synthesis, increased level of tetrapyrrole intermediates, and delayed chloroplast development. Genetic analysis demonstrated that the phenotype of ygl1 was caused by a recessive mutation in a nuclear gene. The ygl1 locus was mapped to chromosome 5 and isolated by map-based cloning. Sequence analysis revealed that it encodes the Chl synthase and its identity was verified by transgenic complementation. A missense mutation was found in a highly conserved residue of YGL1 in the ygl1 mutant, resulting in reduction of the enzymatic activity. YGL1 is constitutively expressed in all tissues, and its expression is not significantly affected in the ygl1 mutant. Interestingly, the mRNA expression of the cab1R gene encoding the Chl a/b-binding protein was severely suppressed in the ygl1 mutant. Moreover, the expression of some nuclear genes associated with Chl biosynthesis or chloroplast development was also affected in ygl1 seedlings. These results indicate that the expression of nuclear genes encoding various chloroplast proteins might be feedback regulated by the level of Chl or Chl precursors.
Chlorophyll (Chl) molecules universally exist in photosynthetic organisms. They perform essential processes of harvesting light energy in the antenna systems and driving electron transfer in the reaction centers (Fromme et al., 2003). Their metabolism has been extensively studied in various organisms by both biochemical (Pontoppidan and Kannangara, 1994) and genetic approaches (Bollivar et al., 1994a; Nakayashiki et al., 1995; Tanaka et al., 1998). Early enzymatic steps of Chl biosynthesis in converting 5-aminolevulinate acid (ALA) to protoporphyrin IX (Proto IX) are shared with the heme biosynthesis pathway. Many essential data regarding the identity of the associated enzymes were obtained from studies on nonphotosynthetic organisms such as Escherichia coli (Narita et al., 1996). The later steps of Chl biosynthesis are common with bacteriochlorophyll a biosynthesis (Porra, 1997; Suzuki et al., 1997). Directed mutational analysis with a photosynthetic bacterium, Rhodobacter capsulatus, provided abundant information on the genes involved in bacteriochlorophyll biosynthesis (Bollivar et al., 1994b), and homologous genes have been isolated from oxygenic plants (Jensen et al., 1996). With the recent identification of 3,8-divinyl protochlorophyllide (Pchlide) a 8-vinyl reductase, all genes required for Chl biosynthesis have been identified in higher plants (Nagata et al., 2005). Analysis of the complete genome of Arabidopsis (Arabidopsis thaliana) shows that there are at least 27 genes encoding 15 enzymes involved in Chl biosynthesis from glutamyl-tRNA to Chl b (Nagata et al., 2005).
Chl synthase is believed to be bound to the thylakoid membranes and to catalyze prenylation of chlorophyllide (Chlide) with geranygeranyl diphosphate (GGPP) or phytyl diphosphate (PhyPP), the last step of Chl biosynthesis (Rüdiger et al., 1980; Soll and Schultz, 1981; Soll et al., 1983). This step is essential for the accumulation of Chl a (Eichacker et al., 1990, 1992) and is likely essential for stable assembly of other thylakoid membrane components (Paulsen et al., 1990; Rüdiger, 1992, 1993). Detailed investigations of the properties of Chl synthase became feasible after demonstrating that the bacteriochlorophyll synthase gene (bchG) of R. capsulatus encodes bacteriochlorophyll synthase (Bollivar et al., 1994b). The recombinant enzyme, produced by expression of R. capsulatus bchG in E. coli, specifically accepted bacteriochlorophyllide but not Chlide, while expression of the Chl synthase gene (CHLG) from Synechococcus sp. PCC 6803 yielded a Chl synthase that accepted Chlide but not bacteriochlorophyllide. Both enzymes exhibited a marked preference for PhyPP over GGPP (Oster et al., 1997), however, Arabidopsis Chl synthase preferred GGPP as the substrate (Oster and Rüdiger, 1997). The G4 gene (later named CHLG) of Arabidopsis encoding Chl synthase had previously been isolated and heterologously expressed in E. coli (Gaubier et al., 1995; Oster et al., 1997). Further characterization of the heterologously expressed Chl synthase from oat (Avena sativa) revealed the importance of Cys and Arg residues in the enzyme and a requirement for Mg2+ ions for its activity (Schmid et al., 2001). Random sequence analysis of EST cDNAs from rice (Oryza sativa) yielded a putative Chl synthase homolog (Lopez et al., 1996; Scolnik and Bartley, 1996), however, the biochemical properties and physiological functions remain unknown.
Mutants deficient in Chl synthesis have been identified in a number of multicellular plant species (Killough and Horlacher, 1993; Falbel and Staehelin, 1996; Falbel et al., 1996). Their genetic characteristics, microstructures, absorption spectra, fluorescence, and physiological properties have been studied systematically (Killough and Horlacher, 1993; Falbel and Staehelin, 1996; Falbel et al., 1996; Havaux and Tardy, 1997). However, mutants in the Chl synthase have not been reported. In this study, we isolated a rice Chl-deficient mutant, yellow-green leaf1 (ygl1). The mutant plant exhibits a yellow-green leaf phenotype, decreased level of Chl, and delayed chloroplast development. Map-based cloning of the mutation resulted in the identification of the YGL1 gene, which has a sequence similarity to the Chl synthase gene. The ygl1 mutant carries a missense mutation (C to T, at residue 592), resulting in an amino acid change (Pro-198 to Ser) in the active region of the enzyme. The mutant phenotype was complemented by transformation with the wild-type gene. Esterification activity of the mutant recombinant protein expressed in E. coli was reduced compared to that of wild type. This study reports the identification of the first mutant of the last step of Chl biosynthesis in higher plants.
RESULTS
The ygl1 Mutant Has Reduced Chl Accumulation and Delayed Chloroplast Development
The ygl1 mutant was a spontaneous mutant isolated from indica rice ‘Zhenhui 249’, which exhibited a yellow-green leaf phenotype. The ygl1 mutant was slightly smaller than wild type throughout the developmental stage (Fig. 1, A–C) and exhibited reduced levels of Chl a/b as well as carotenoid (Car) content (Table I). Leaves of the ygl1 mutant had 20% to 70% reduction of Chl, and 30% to 40% reduction of Car levels compared to those in wild type at different stages, with the most significant differences detectable in 4-week-old plants. The Chl a/b ratio appeared highest at the seedling stage, due likely to the potential of Chl b synthesis in suffering a more severe decline than Chl a. The Chl a/b ratio then declined to eventually reach the wild-type level. Together this suggests that the ygl1 mutant exhibited delayed greening during photomorphogenesis because of slow rates of Chl accumulation. Eventually, mutant plants accumulated substantial quantities of Chl, reaching almost the wild-type levels and becoming slightly yellow with the maturation of leaves.
Figure 1.
Phenotypic characterization of the rice ygl1 mutants. A, Four-week-old plants. B, Ten-week-old plants. C, Fifteen-week-old plants. D, Chloroplasts of the first leaf from top to base have abundant, well-ordered stacks in 4-week-old wild type. E, Chloroplasts of the first leaf in 4-week-old ygl1 mutant have few or no membrane stacks and only occasional long, parallel, and unstacked membranes in worse order than in wild type. Examples of chloroplast (Cp), plastoglobule (Pg), and mitochondrion (Mt). Bar equals 0.5 μm.
Table I.
Pigment contents in leaves of wild-type and ygl1 mutant, in mg g−1 fresh weighta
| Growth Stage | Genotype | Total Chl | Chl a/b Ratio | Car |
|---|---|---|---|---|
| 4 weeks old | Wild type | 4.84 ± 0.16 | 3.63 ± 0.01 | 0.54 ± 0.04 |
| ygl1 | 1.53 ± 0.01 | 7.91 ± 0.01 | 0.35 ± 0.01 | |
| 10 weeks old | Wild type | 3.55 ± 0.29 | 3.46 ± 0.02 | 0.67 ± 0.04 |
| ygl1 | 2.60 ± 0.03 | 4.38 ± 0.08 | 0.40 ± 0.00 | |
| 15 weeks old | Wild type | 3.32 ± 0.08 | 3.13 ± 0.04 | 0.57 ± 0.04 |
| ygl1 | 2.97 ± 0.06 | 3.35 ± 0.01 | 0.40 ± 0.01 |
Chl and Car were measured in acetone extracts from second leaf of different growth stages from top. Values shown are the mean sd (±sd) from five independent determinations.
To investigate how the ygl1 mutation affects chloroplast development, we compared the ultrastructures of plastids in the ygl1 mutant and wild-type plants at different developmental stages using transmission electron microscopy. Granal stacks in the ygl1 mutant appeared less dense (Fig. 1E) and lacked granal membranes compared to those of wild type (Fig. 1D) in developing leaves. Granal development in the ygl1 mutant was slower than that of wild type, and granal membranes in the ygl1 mutant increased when the leaf became mature (data not shown).
The ygl1 Locus Maps to a Putative Gene Encoding Chl Synthase on Chromosome 5
For genetic analysis of the ygl1 mutant, four F2 populations were constructed from the crosses between the ygl1 mutant and ‘PA64’, ‘W002’, ‘USSR5’, and ‘02428’. All F1 plants from the four crosses displayed wild-type phenotype, and their F2 progenies all showed a segregation ratio of 3:1 (green:yellow-green plants, χ2 < χ20.05 = 3.84; P > 0.05; Table II). Therefore, the yellow-green leaf phenotype in the ygl1 mutant was controlled by a single recessive nuclear gene.
Table II.
Segregation of F2 populations from four crosses
| Cross | ygl1a/PA64 | ygl1/W002 | ygl1/USSR5 | ygl1/02428 |
|---|---|---|---|---|
| Numbers of green plantsb | 263 | 152 | 810 | 155 |
| Numbers of yellow plants | 89 | 43 | 251 | 48 |
| Total numbers | 352 | 195 | 1,061 | 203 |
| χ2 | 0.50 | 0.75 | 0.95 | 0.13 |
| Pc | 0.49 | 0.41 | 0.34 | 0.72 |
The female partner for the cross.
Green plants and yellow plants were determined by visual inspection.
P > 0.05 considered as significant.
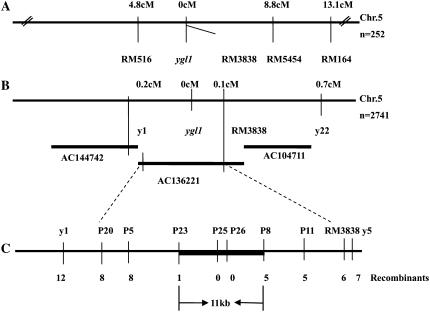
To map the ygl1 locus, an F2 mapping population was generated from a cross between the ygl1 mutant with ‘PA64’. The ygl1 gene was mapped to an interval between markers RM516 and RM164 on chromosome 5 (Fig. 2A). Comparison of the chromosomal locations and leaf color phenotypes indicated that ygl1 was a novel gene and different from the previously identified genes related to leaf color alteration (Nagato and Yoshimura, 1998). To narrow down the search for a candidate gene affected in ygl1 mutant plants, a larger F2 mapping population consisting of more than 12,000 plants, of which 2,741 segregants showed the ygl1 mutant phenotype were used for fine mapping. Three simple sequence repeat (SSR) markers and seven cleaved-amplified polymorphic sequence (CAPS) markers were developed (Table III) between markers RM516 and RM164. The ygl1 locus was mapped to an 11-kb DNA region between the two CAPS markers P23 and P8 on a single bacterial artificial chromosome (BAC), AC136221 (Fig. 2, B and C). Within this region, two open reading frames (ORFs) were predicted using the program FGENESH 2.2 (www.softberry.com). The first ORF encoded a putative Chl synthase that shows a high similarity to the oat Chl synthase gene, an enzyme required for Chl a biosynthesis (Schmid et al., 2001). The second ORF was the Osem gene, encoding a protein similar to embryonic abundant protein. To define the molecular lesions of the ygl1 mutant, both candidate ORFs were amplified by reverse transcription (RT)-PCR from the ygl1 mutant and wild-type plants, respectively, and sequenced. Comparison of the sequences revealed that only the first ORF was altered, exhibiting a single nucleotide mutation at codon 592 (T → C) in the eighth exon, which resulted in an amino acid change from Pro-198 to Ser (Fig. 3A). Therefore, we tentatively designated the first ORF as the YGL1 gene.
Figure 2.
Map-based cloning of the ygl1 locus. The map was constructed based on the publicly available sequence of rice chromosome 5. Seven CAPS markers (P5, P8, P11, P20, P23, P25, and P26) were produced during this study, while three SSR markers RM516, RM5454, and RM3838 were obtained from the public database, and SSR markers y1, y5, and y22 were developed in the work. A, The ygl1 locus was mapped to a region between markers RM516 and RM5454 on the long arm of rice chromosome 5 (Chr.5) with 252 recessive individuals. B, Fine mapping of the ygl1 locus between y1 and RM3838 from a segregating population of 2,741 recessive individuals. Two BAC contigs (AC144742 and AC136221) cover the ygl1 locus. C, The ygl1 gene was narrowed down to an 11-kb genomic DNA region between the CAPS markers P23 and P8, and cosegregated with P25 and P26.
Table III.
The PCR-based molecular markers designed for fine mapping
| Type of Marker | Marker | Primer Pairs | Fragment Size | Restriction Enzyme | Originated BAC |
|---|---|---|---|---|---|
| bp | |||||
| SSR | y1 | 5′GCGGTTGAAGGCGTCGTA3′ | 116 | AC144742 | |
| 5′AGGGTGCTGAGTCACAATAGGT3′ | |||||
| y5 | 5′CCCCAAACTAATTTCCTCCT3′ | 100 | AC136221 | ||
| 5′ACATCTGTAACCAATCCTCCC3′ | |||||
| y22 | 5′CTGCCCTTGAATAATGACG3′ | 177 | AC104281 | ||
| 5′GCGACTGATCGGTACTCCT3′ | |||||
| CAPS | P5 | 5′GGCTAGTTATGGGTTAGAGGGTA3′ | 709 | XbaI | AC136221 |
| 5′TCCCTTTTCAAATCACACGA3′ | |||||
| P8 | 5′CGTGCAGGTTGTGTGACTCT3′ | 922 | TaqI | AC136221 | |
| 5′GTTACAGAGCCAGCCAGGAG3′ | |||||
| P11 | 5′TTTGATCGCCGTCATGTTTA3′ | 934 | HapII | AC136221 | |
| 5′CCGGTGGTGAAGTCGTAGAT3′ | |||||
| P20 | 5′GCAGTTATTGGAAGTCAGC3′ | 685 | NsiI | AC136221 | |
| 5′ATTACATCTACGTGCAAAGTC3′ | |||||
| P23 | 5′CAACCACCTCAAGCTCTTT3′ | 197 | TaqI | AC136221 | |
| 5′ATATTTCCTTCCCTACCCA3′ | |||||
| P25 | 5′GGAATTAGTGCCACCAAAC3′ | 834 | StyI | AC136221 | |
| 5′GGGAATCAACAAGAAACGA3′ | |||||
| P26 | 5′CCTCATTTTCCTTGGAGCAG3′ | 740 | MfeI | AC136221 | |
| 5′TCTTCGCACCTAAGGTCACA3′ |
Figure 3.
Sequence analysis of YGL1 homologs. A, Alignment of the derived amino acid sequence of rice with published Chl and bacteriochlorophyll synthase sequences. Identical residues are boxed in black, similar residues are highlighted in gray. The Pro-198 to Ser change is indicated with an asterisk at the mutation site of ygl1. Domain II, suggested to be the binding site of the polyprenyl PP, is underlined. GenBank accession numbers for the respective protein sequences are rice (OsYGL1, ABO31092); oat (AsCHLG, AJ277210); Arabidopsis (AtCHLG, At3G51820); Synechooystis sp. PCC 6803 (SCHLG, BA000022); R. capsulacus (RcbchG, CAA77532); Rhodobacter sphaeroides (RsbchG, CP000143); Heliobacillus mobilis (HmbchG, AAC84024); and Chloroflexus aurantiacus (CabchG, AAG15227). B, A phylogenetic tree representing alignment of YGL1 proteins. The rooted tree using percentage identities is based on a multiple sequence alignment generated with the program DNAMAN. Scale represents percentage substitution per site.
Searching the rice genome database revealed that YGL1 is a single-copy gene with a 1,131-bp ORF. The coding region of YGL1 gene is comprised of 15 exons and encodes a 376-amino acid protein with the molecular mass of approximately 41 kD. YGL1 contains an apparent chloroplast-targeting sequence of 47 amino acids at its N terminus. Multiple amino acid sequence alignments showed that YGL1 had a significant similarity to the representatives from particular classes of Chl and bacterchlorophyll synthases from different organisms (Fig. 3A). For example, YGL1 is 88.39% identical to Chl synthase from oat (Schmid et al., 2001), 74.68% identical to the Arabidopsis G4 gene product (Oster and Rüdiger, 1997), and 53.94% identical to the Synechocystis sp. PCC 6803 enzyme (Kaneko et al., 1995). Moreover, YGL1 is also homologous (23%–30%) to various bacteriochlorophyll synthases (Bollivar et al., 1994a, 1994b; Lopez et al., 1996; Xiong et al., 1998; Addlesee et al., 2000).
We then analyzed the possible phylogenetic relationships between YGL1 and its related proteins from higher plants and cyanobacteria (Fig. 3B). The result indicated that rice YGL1 was more closely related to Chl synthase from the monocotyledon plant oat than to those of other species. Not surprisingly, YGL1 has a phylogenetically much closer relationship to Chl synthases of the higher plant species than to bacteria proteins. In addition, it is interesting to note that bacteriochlorophyll synthases lack a motif (WAGHDF-197) that exists only in the Chl synthase (Supplemental Fig. S1). Analysis with the transmembrane calculation programs (Nilsson et al., 2002) revealed that the ygl1 mutation site occurred at or close to the end of a transmembrane helix (data not shown).
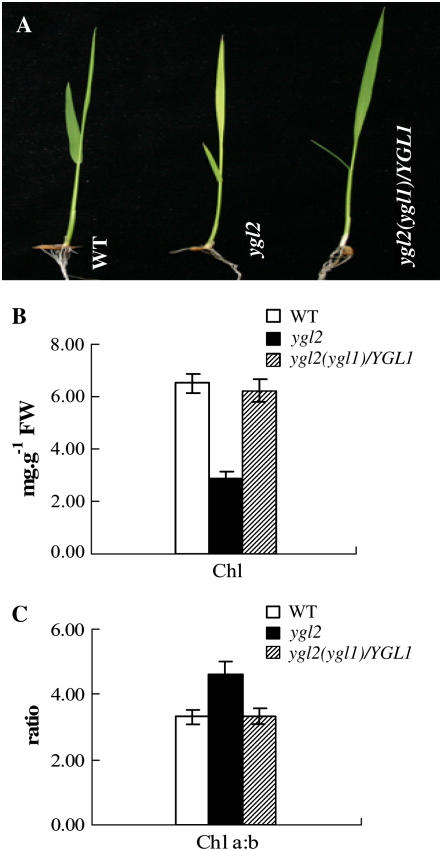
The identity of ygl1 was subsequently confirmed by genetic complementation experiments (Fig. 4A). The color of leaves, the levels of Chl, and the ratio of Chl a/b were all restored to levels of wild-type plants upon transformation with the YGL1 gene (Fig. 4, B and C). Therefore, this confirms that observed abnormal phenotypes of the ygl1 mutant plants resulted from mutation of the YGL1 gene.
Figure 4.
Complementation of the ygl1 mutant by wild-type gene. The japonica rice ygl2 with ygl1 allele was used as transforming material (see “Materials and Methods”). A, Phenotypes of the wild type, the ygl2 mutant, and the transgenic plant, ygl2 (ygl1)/YGL1. Photographs were taken 2 weeks after sowing. B, Total Chl levels of wild type, ygl2 mutant, and ygl2 (ygl1)/YGL1. Chl was extracted from the second leaf of 2-week-old plants. C, Chl a/b ratio calculated from B. Error bars represent sd (±sd), and representative data from three independent experiments are presented.
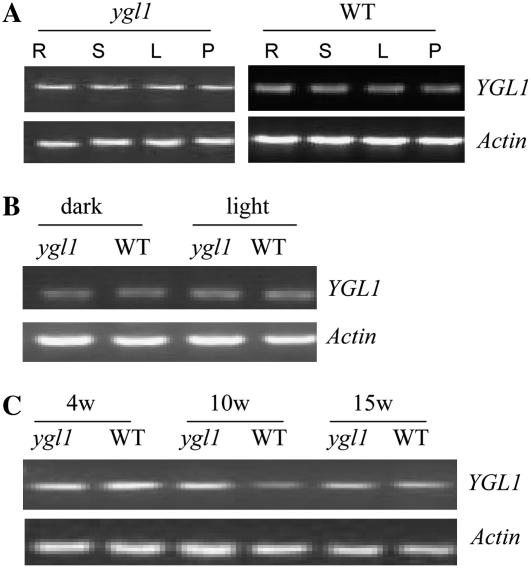
YGL1 mRNA Expression Level Is Not Affected by the ygl1 Mutation of YGL1
We compared the level of YGL1 transcript in ygl1 mutant and wild-type plants using RT-PCR. Figure 5A showed that YGL1 mRNA was expressed at similar levels in root, leaf sheaths, leaves, and young panicles in both the ygl1 mutant and wild type. We also examined the effect of light and dark growth conditions on the expression of YGL1. No change in transcript levels was observed when ygl1 or wild-type plants were grown under light or dark conditions (Fig. 5B). Furthermore, no significant differences of YGL1 mRNA levels were observed in the mutant compared to wild type from early to mature stages (Fig. 5C). These results indicate that the missense mutation of ygl1 does not affect its own mRNA expression.
Figure 5.
Expression analysis of YGL1 by RT-PCR. Total RNA was extracted from root (R), leaf sheath (S), leaf (L), and young panicle (P) of wild type and ygl1 mutant. A, Expression patterns of YGL1 in root (R), leaf sheath (S), leaf (L), and young panicle (P) of wild type and ygl1 mutant. B, YGL1 expression in wild-type and the ygl1 mutant leaves of 2-week-old plants grown in dark or under light. C, YGL1 expression in wild-type and ygl1 mutant leaves of 4-, 10-, and 15-week-old plants. RT-PCR was repeated three times and representative results (25 cycles) are shown. Actin was amplified as a control.
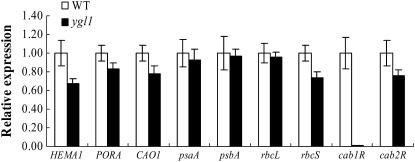
We next addressed the question of whether the ygl1 mutation affected the transcript of other genes associated with Chl biosynthesis, chloroplast development, or photosynthesis. Analysis of mRNA levels using real-time PCR showed that the expression of genes involved in Chl biosynthesis, such as glutamyl tRNA reductase (HEMA1), was reduced by about 40%, and Chlide a oxygenase1 (CAO1) and NADPH:Pchlide oxidoreductase (PORA) were slightly reduced in ygl1 mutant seedlings compared with wild type (Fig. 6). Interestingly, the expression of cab1R, which encodes the light-harvesting Chl a/b-binding protein of PSII (Matsuoka, 1990), was severely suppressed, whereas another cab gene, cab2R, showed only slightly decreased mRNA levels in the ygl1 mutant. The expression levels of plastid genes, psaA and psbA encoding two reaction center polypeptides, and rbcL encoding the large subunit of Rubisco, were not significantly reduced in the ygl1 mutant. However, the expression of the nuclear rbcS gene encoding the small subunit of Rubisco (Kyozuka et al., 1993) was slightly decreased in the ygl1 mutant (Fig. 6). Taken together, it is likely that that the ygl1 mutation affected the transcript of most nuclear genes, such as cab1R, HEMA1, CAO1, etc., but not the expression of plastid-encoded genes including psaA, psbA, and rbcL in the ygl1 mutant.
Figure 6.
Expression analysis of genes associated with Chl biosynthesis, photosynthesis, or chloroplast development by real-time PCR. Total RNA was extracted from leaves of 4-week-old (4w) plants. Actin was amplified as a control. Error bars represent sd (±sd) and representative data from three independent experiments are presented.
Single Amino Acid Change Causes a Reduction in Chl Synthase Activity
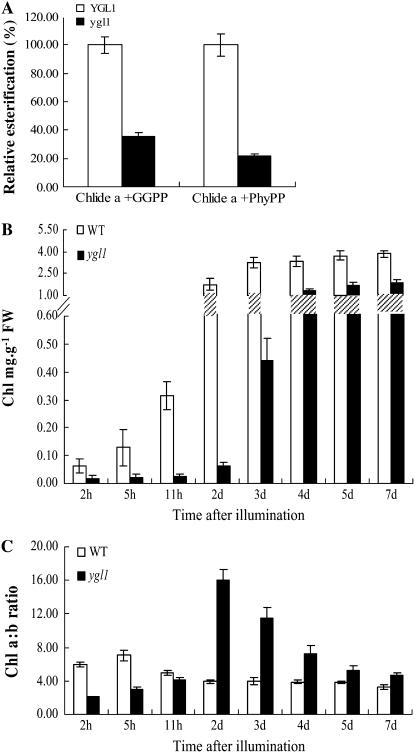
We then examined whether the Pro-198 to Ser amino acid substitution in ygl1 impaired enzymatic function of the ygl1 mutant. An in vitro assay was used to compare the esterification activity of the recombinant Chl synthase enzymes produced from E. coli using Chlide a along with two different substrates, GGPP and PhyPP (Oster et al., 1997). The result showed that the esterification activity of the recombinant mutant ygl1 exhibited approximately 35.22% and 21.75% esterification of Chlide a with GGPP and PhyPP, respectively, compared to wild-type recombinant enzyme YGL1, whose activity was set at 100% (Fig. 7A).
Figure 7.
Activity of recombinant proteins and time course of Chl accumulation. A, Activity of recombinant YGL1 and ygl1 proteins in E. coli. Total enzyme activity was determined using the extracts from the induced bacterial cells with Chlide a plus PhyPP or Chlide a plus GGPP. Equal amounts of protein were used. B, Time course of Chl accumulation. C, Time course of Chl a/b ratio based on the results in B. The seedlings were grown in darkness for 1 week, Chl content and Chl a/b ratio was measured after exposure to white light for various times as indicated. Error bars represent sd (±sd) from three independent experiments are presented.
The rate of Chl accumulation was next compared in the ygl1 mutant and wild-type seedlings. The seedlings were grown in darkness for 1 week and Chl content and Chl a/b ratio were measured after exposure to white light at various times. The results showed that the rate of Chl accumulation was slower in the ygl1 mutant than in the wild type (Fig. 7B). The ratio of Chl a/b was lower in the ygl1 mutant than in wild type initially, and it became higher after 2 d. Notably, the peak value at 2 d in ygl1 mutant was substantially more than the peak value of wild type at 5 h (Fig. 7C). One possible explanation is that Chl a synthesis becomes limiting and Chl a preferentially assembles reaction centers (RCs) in the ygl1 mutant; once the RCs were no longer incorporating the majority of Chl a, Chl b was produced in significant amounts only when there was leftover Chl a (Falbel and Staehelin, 1996; Falbel et al., 1996). These data suggest that aberration of Chl synthase function results in decrease of Chl a synthesis in the ygl1 mutant.
Reduced Activity of Chl Synthase Results in Accumulation of Intermediates of Chl Biosynthesis
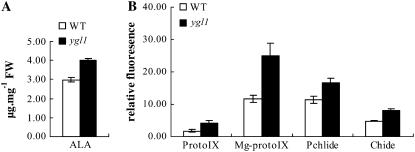
When angiosperm plants were grown in dark conditions, Pchlide accumulated instead of Chl and plants had an etiolated phenotype (Schoch et al., 1977; Schoch, 1978). Mock and Grimm (1997) characterized transgenic plants with deficiencies in coproporphyrinogen oxidase and uroporphyrinogen decarboxylase, two preceding enzymes in the metabolic pathway of Chl synthesis. These plants accumulated their respective substrates, uroporphyrin(ogen) and coproporphyrin(ogen), in young leaves up to 100-fold times the levels in control plants and exhibited necrotic lesions. Since the ygl1 mutant had deficient Chl synthase activity, Chlide was predicted to accumulate in ygl1 mutant plants. Not surprisingly, compared to wild-type plants, ygl1 mutants accumulated higher levels of Childe and other intermediates, including ALA (Fig. 8A), Proto IX, Mg-Proto IX, and Pchlide, in leaves of seedlings (Fig. 8B). Together, these results showed that Chl synthase plays a critical role in Chl biosynthesis.
Figure 8.
Analysis of Chl intermediates in wild-type and ygl1 mutant. Chl intermediates were measured in second leaf from 2-week-old wild-type and ygl1 mutants. A, Levels of ALA. B, Relative fluorescence of Proto IX, Mg-proto IX, Pchlide, and Chlide. Error bars represent sd (±sd) and representative data from three independent experiments are presented.
DISCUSSION
Chl synthase has been the subject of thorough investigation (for review, see Suzuk, 1997; Willows, 2003). However, to our knowledge, no corresponding Chl synthase mutant has been found in higher plants. In this study, we have identified and functionally characterized the Chl synthase mutant, ygl1 of rice, at the molecular level.
Lopez et al. (1996) described bacteriochlorophyll and Chl synthases as belonging to the family of polyprenyltransferases, and suggested a homologous region (domain II) to be the binding site of the polyprenyl PP. Multialignment analysis showed that this region spans residues 140 to 162 in the YGL1 sequence (Fig. 3A). Oster found that the esterification activity of oat Chl synthase was lost when the residue of His-197 (His-195 in YGL1) was mutated, presumably due to His-197 possible overlap with the Mg2+-binding site from Chlide (personal communication). A point mutation (Pro-198 to Ser) in YGL1 was found at the highly conserved Pro-198, which compromised the esterification activity of Chl synthase (Fig. 7A). Analysis of YGL1 derived amino acid sequence showed that Pro-198 was in proximity to a motif (WAGHDF-197) specifically found only in Chl synthases, but not in bacteriochlorophyll synthases (Supplemental Fig. S1), which differ, in part, based on preference of substrates, either Chlide (targeted by Chl synthases) or bacteriochlorphyllide (for bacteriochlorophyll synthases; Oster et al., 1997). Therefore, the importance of the essential Pro-198 residue in YGL1 could be attributed to its location in or proximity to the binding site of Chlide. Future studies to pursue this possibility would help further elucidate the substrate specificity and targeting mechanisms between the synthase family members.
In this study, the ygl1 mutant seedlings displayed a yellow-green phenotype and became green with leaf Chl accumulation at the mature stage (Table I). The Car content was significantly lower in the mutant plants compared to wild type, even in older leaves in which the Chl content was the same as wild type (Table I). This result might be related to the parallel degradation of pigments and pigment-binding proteins of the photosynthetic apparatus (Cunningham and Gantt, 1998; Papenbrock et al., 2000).
Mutation of the YGL1 gene reduced Chl levels and resulted in a yellow-green phenotype more or less specific to younger plants. Why the ygl1 mutation affects Chl biosynthesis most dramatically in the early developmental stage but is restored in later stages is not yet completely understood. One possible explanation is that there might be other Chl synthase homologs with redundant functional activities in later stages. However, no other rice Chl synthase genes were identified from a survey of the rice genome database (International Rice Genome Sequencing Project, 2005). These results were consistent with those of Gaubier et al. (1995) and Schmid et al. (2001), which showed that the Chl synthase sequence represented a single-copy gene in Arabidopsis and oat by Southern- and northern-blot analysis. Since we did not find significant differences in transcription level of the YGL1 gene at the different development stages, one possibility is that the enzyme is regulated at the translational level. This hypothesis remains to be tested directly.
Moreover, the delayed chloroplast development might lead to a slow accumulation of Chl in ygl1 mutant seedling leaves. Chl synthase was proposed to localize to the thylakoid membranes where esterification of Chlide a with phytol or earlier alcohol precursors take place (Block et al., 1980; Rüdiger et al., 1980; Soll and Schultz, 1981). Soll et al. (1983) showed that the Chl synthase in chloroplasts is more stable than those from etioplasts. This implied that Chl a biosynthesis catalyzed by Chl synthase was associated with chloroplast development (El-Saht, 2000; Biswal et al., 2003). Therefore, Chl deficiency caused by the ygl1 mutant might be due to delayed formation of thylakoid membranes, and the underdeveloped chloroplast led to the decease of Chl accumulation in ygl1 seedlings stage.
Previous reports indicated that the transcript of the Arabidopsis G4 gene was detected only in green or greening tissues, and its expression was not strictly light dependent, while oat CHLG gene was constitutively expressed (Gaubier et al., 1995; Schmid et al., 2001). Our experiments showed that rice YGL1 was constitutively expressed, which is consistent with trends observed for oat CHLG (Schmid et al., 2001). Another notable observation was the effect of the YGL1 mutation on the mRNA expression of some genes associated with Chl biosynthesis or chloroplast development. Among the genes examined, we found that the expression of most nuclear genes, including cab1R, HEMA1, CAO1, and PORA, were reduced at different levels, whereas the plastid-coded genes, such as psaA, psbA, and rbcL, were not significantly influenced in ygl1 seedlings (Fig. 6). This suggests, then, that the expression of the plastid-encoded genes might be regulated at the level of translation rather than transcription.
In the ygl1 mutant, the transcript level of cab1R gene was severely impaired and markedly different from that of cab2R, which was only slightly decreased at the young seedling stage (Fig. 6), indicating that both are differentially regulated (Matsuoka, 1990). Although the expression of the nuclear multigene (cab) family was a marker for chloroplast development and tightly controlled by both light and plastid signals, including a circadian clock, hormones, and Suc levels (Flores and Tobin, 1986; Karlin-Neumann et al., 1988; Millar and Kay, 1996; Dijkwel et al., 1997), it was not clear whether the expression of the cab gene might be an indirect consequence of Chl deficiency, or, even more plausibly, of the feedback regulation by plastid signals from the accumulation of higher levels of Chl precursors in the ygl1 mutant. Elucidation of the mechanism would provide greater understanding toward cellular signaling and feedback mechanisms between nucleus and plastids.
CAO was previously considered to be the only enzyme responsible for Chl b synthesis. Recombinant CAO had been shown to convert Chlide a into Chlide b, most likely by a two-step oxygenation (Oster et al., 2000), with Chl synthase adding a hydrophobic phytol tail to produce Chl a and Chl b (Oster et al., 1997). The ratio of Chl a/b was reported to correlate with CAO mRNA levels in Arabidopsis (Harper et al., 2004) and Dunaliella salina (Masuda et al., 2002, 2003b). However, our studies show that CAO mRNA was slightly decreased and the Chl a/b ratio was largely increased (from 3.63–7.91; Table I) in the ygl1 mutant, suggesting that CAO activity might be regulated at the posttranscriptional level (Tanaka et al., 2001).
Chl a is required for the formation of photosynthetic reaction centers and light-harvesting complexes, and Chl b is exclusively located in the light-harvesting pigment protein complexes of PSI and PSII. An appropriate ratio of Chl a/b is critical in the regulation of photosynthetic antenna size (Jansson, 1994; Oster et al., 2000; Tatsuru et al., 2003). However, partial block in Chl a biosynthesis caused a decrease of the Chl content and an increase in the ratio of Chl a/b in young leaves of the ygl1 mutant (Falbel and Staehelin, 1996), indicating that the total number of photosystems decreased and light-harvesting antenna complexes might be lower than that of wild type. Further characterization of the YGL1 gene could provide deeper insight into understanding the relationship between the biosynthesis of Chl a and Chl b, and between the biosynthesis of Chl, Cars and proteins, the regulation of photochemical reactions, as well as the assembly of the thylakoid membranes and chloroplast development.
MATERIALS AND METHODS
Plant Materials
The rice (Oryza sativa) yellow-green leaf mutant (ygl1) was isolated from indica ‘Zhenhui249’. The ygl1 was crossed with ‘PA64’ to construct the F2 mapping population. ‘PA64’ has a major genetic background of indica and minor gene flows from javanica (Bao et al., 2005).
Genetic Analysis and Marker Development
Genomic DNA was extracted and analyzed for cosegregation using available SSR (McCouch et al., 2002) from F2 plants. New SSR markers were developed based on the Nipponbare genome sequence information from the National Center for Biotechnology Information database and by searching for simple repeat sequences with the SSRIT program (Temnykh et al., 2000). CAPS markers were developed on comparisons of original or CAPS length by using SNP2CAPS soft (Thiel et al., 2004) between the indica var. ‘PA64s’ (J. Yu, unpublished data) and indica var. ‘9311’ according to the data published in http://www.ncbi.nlm.nih.gov.
Sequence Analysis
The full-length CHLG protein sequences were retrieved from GenBank and used for phylogenetic analysis according to the methods described by Li et al. (2003). The signal peptide was predicted with SignalP version 2.0 (Nielsen and Krogh, 1998). Phylogenetic analysis and multiple sequence alignment were conducted by using DNAMAN version 6.0 (Lynnon Biosoft). The residue-specific hydropathy index was predicted by using the transmembrane calculation programs (Nilsson et al., 2002).
Complementation of the ygl1 Mutant
As Agrobacterium-mediated transformations are difficult to perform in indica rice, the ygl1 gene was also transferred to Wuyunjing 8 (spp. japonica) by five rounds of backcrosses with Wuyunjing 8 and self crossed for five generations. We obtained an isogenic line with ygl1 allele in japonica genetic background and named it as ygl2, which was used as transforming material.
For complementation of the ygl1 mutation, a full-length cDNA fragment encoding YGL1 was amplified by RT-PCR using the primers 5′-AACTGCAGAGTCTCCAATGGCCACCTC-3′ and 5′-GGACTAGTGCTTTCATCAGTGGCTGGTT-3′ from the wild type. The primers incorporated a PstI site at the N-terminal end and a SpeI site at the C-terminal end of the ORF. PCR products were cloned into the pMD18-T vector (TaKaRa). Then the YGL1 cDNA fragment from wild type was digested with PstI and SpeI and ligated into the PstI and SpeI sites of a binary vector pCUbi1390 (T. Lu, unpublished data) harboring a hygromycin-resistant gene. The resulting pCUYGL1 plasmid, which contained the YGL1 coding sequence driven by the ubiquitin promoter, was transformed into Agrobacterium tumefaciens strain EHA105 by electroporation, and transformed to japonica rice ygl2 for complementation testing according to a published method (Hiei et al., 1994).
Analysis of RT-PCR and Real-Time PCR
Total RNA was extracted from leaves, leaf sheaths, young panicles, and roots according to the method described by Wadsworth et al. (1988). cDNA synthesis was performed using 5 μg total RNA for each sample. RNA was treated in 1× buffer with 5 units of DNase I (MBI Fermentas) added to the reaction and incubated for 30 min at 37°C. The reaction was stopped by adding 1 μL of 25 mm EDTA, followed by 10 min incubated at 65°C. For RT-PCR, first-strand cDNA was reverse transcribed from total RNA with oligo (dT) and avian myeloblastosis virus reverse transcriptase (TaKaRa). Amplification of ygl1 and YGL1 cDNA (GenBank accession: EF432576) was carried out with specific primers (forward primer, 5′-CAGTCTCCAATGGCCACCT-3′; reverse primer, 5′-TGCTTTCATCAGTGGCTGGT-3′). PCR products were cloned into the pMD18-T vector (TaKaRa). The level of gene expression was analyzed by real-time PCR, and included HEMA1 (GenBank accession: J013000F15) for glutamyl-tRNA reductase, PORA (GenBank accession: NM197169) for Pchlide oxidoreductase, CAO1 (GenBank accession: J013116K15) for Chlide a oxygenase, cab1R (X13908) and cab2R (X13909) for Chl a/b-binding protein from rice, psaA (AAS46121) and psbA (GenBank accession: AAS46104) for two reaction center polypeptides, rbcL (Hirai et al., 1985; GenBank accession: AAS46127) for large subunit of Rubisco, and rbcS (Wanner and Gruissem, 1991; GenBank accession: X07515). The sequences of the PCR primers are as follows: HEMA1, 5′ CGCTATTTCTGATGCTATGGGT 3′, 5′ TCTTGGGTGATGATTGTTTGG 3′; PORA, 5′ TGTACTGGAGCTGGAACAACAA 3′, 5′ GAGCACAGCAAAATCCTAGACG 3′; CAO1, 5′ GATCCATACCCGATCGACAT 3′, 5′ CGAGAGACATCCGGTAGAGC 3′; cab1R, 5′ AGATGGGTTTAGTGCGACGAG 3′, 5′ TTTGGGATCGAGGGAGTATTT 3′; cab2R, 5′ TGTTCTCCATGTTCGGCTTCT 3′, 5′ GCTACGGTCCCCACTTCACT 3′; psaA, 5′ GCGAGCAAATAAAACACCTTTC 3′, 5′ GTACCAGCTTAACGTGGGGAG 3′; psbA, 5′ CCCTCATTAGCAGATTCGTTTT 3′, 5′ ATGATTGTATTCCAGGCAGAGC 3′; rbcL, 5′ CTTGGCAGCATTCCGAGTAA 3′, 5′ ACAACGGGCTCGATGTGATA 3′; rbcS, 5′ TCCGCTGAGTTTTGGCTATTT 3′, 5′ GGACTTGAGCCCTGGAAGG 3′. Actin (GenBank accession: X15865) was used for normalization as a control. Primers for Actin: 5′ AGGAAGGCTGGAAGAGGACC3′, 5′ CGGGAAATTGTGAGGGACAT 3′. PCR was carried out in a total volume of 25 μL containing 0.2 μm of each primer and 1XSYBR green PCR master mix (PE Applied Biosystems). Reactions were amplified in a BIO-RAD iCycler as follows: 95°C for 10 min, then 40 cycles of 95°C for 20 s, 60°C for 20 s, and 72°C for 30 s. The 2−ΔΔCT method was used to calculate relative changes in gene expression as described (Livak and Schmittgen, 2001).
Recombinant Enzymes Activity Assays
Both YGL1 and ygl1 full-length cDNAs were isolated by RT-PCR from the total RNA from ygl1 and wild-type leaves with the RT-PCR system (TaKaRa) using primer 1 (5′CGCGGATCCCAGTCTCCAATGGCCACCT3′) and primer 2 (5′CCCAAGCTTTGCTTTCA TCAGTGGCTGGT3′). The primers incorporated a BamHI site at the N-terminal end and a HindIII site at the C-terminal end of the ORF. The PCR products were inserted into pMD18-T vectors and sequenced to obtain the correct clones, pMDYGL1 and pMDygl1. The pMDYGL1 and pMDygl1 plasmids were then digested and cloned into the corresponding site of the bacterial expression vector pET28-a(+) (Novagen) to generate pETYGL1 and pETygl1, sequenced to confirm YGL1 and ygl1 sequences, respectively, then introduced into Escherichia coli BL21 for protein expression. Protein expression and recombinant enzyme activity assays were according to the method as described by Schmid et al. (2001).
Pigment and Chl Precursor Determination
Total Chl and Cars were determined with DU 800 UV/Vis Spectrophotometers (Beckman Coulter) according to the method of Arnon (1949). Determination of ALA content was based on Richard's (1975) methods. The precursors, including Proto IX, Mg-Proto IX, Pchlide, and Chlide, were assayed as described by Santiago-Ong et al. (2001) and Masuda et al. (2003a). Leaves (approximately 30 mg fresh weight) of wild-type and ygl1 mutant were cut and homogenized in 5 mL 9:1 acetone:0.1 m NH4OH, and centrifuged at 3,000g for 10 min. The supernatants were combined and washed successively with an equal volume of hexane three times prior to spectrophotometric analysis. Chl precursors in the acetone phase were quantified with a Hitachi F-4500 fluorescence spectrophotometer using Ex400:Em632 for Proto IX, Ex440:Em633 for Pchlide, Ex440:Em672 for Chlide, and Ex420:Em595 for Mg-Proto.
Transmission Electron Microscopy Analysis
Wild-type and ygl1 mutant leaf samples were harvested from 1-week- and 1-month-old plants grown in a greenhouse at medium light intensity (approximately 150 μmol photons m−2 s−1). Leaf sections were fixed in a solution of 2% glutaraldehyde and further fixed in 1% OsO4. Tissues were stained with uranyl acetate, dehydrated in ethanol, and embedded in Spurr's medium prior to thin sectioning. Samples were stained again and examined with a JEOL 100 CX electron microscope.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number EF432576 (YGL1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. ClustalW alignment of amino acid sequences surrounding the substituted residue of YGL1 paralogs and bacterial Chl synthase orthologs.
Supplementary Material
Acknowledgments
We are grateful to Prof. Wolfhart Rüdiger and Dr. Ulrike Oster at the Botanics Institute of the University of Munich for the Chl synthase activity assay. We thank Dr. Yu Jun at the Beijing Genomics Institute at the Chinese Academy of Sciences for access to the ‘PA64s’ partial genome sequence prior to its publication. We also thank Prof. Sodmergen and Dr. Yingchun Hu at the College of Life Sciences at Peking University for their assistance in electron microscopy analysis, and Dr. Lu Tiegang at the Chinese Academy of Agricultural Sciences for providing the rice transformation vector pCUbi1390.
This work was supported by the National Key Basic Research “973” Program of China (grant nos. 2006CB1017000 and 2006CB100201), by the National “863” Program (grant no. 2006AA100101), and by the Program for Changjiang Scholars and Innovative Research Team in University.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jianmin Wan (wanjm@caas.net.cn).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Addlesee HA, Fiedor L, Hunter CN (2000) Physical mapping of bchG, orf427, and orf177 in the photosynthesis gene cluster of Rhodobacter sphaeroides: functional assignment of the bacteriochlorophyll synthetase gene. J Bacteriol 182 3175–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenol-oxidase in Beta vulgaris. Plant Physiol 24 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao JY, Lee S, Chen C, Zhang XQ, Zhang Y, Liu SQ, Clark T, Wang J, Cao ML, Yang HM, et al (2005) Serial analysis of gene expression study of a hybrid rice strain (LYP9) and its parental cultivars. Plant Physiol 138 1216–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal UC, Biswal B, Raval MK (2003) Protoplastid to chloroplast transformation. In J Kutik, ed, Chloroplast Biogenesis. From proplastid to Gerontoplast. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 19–77
- Block MA, Joyard J, Douce R (1980) Site of synthesis of geranylgeraniol derivatives in intact spinach chloroplasts. Biochim Biophys Acta 631 210–219 [DOI] [PubMed] [Google Scholar]
- Bollivar DW, Suzuki JY, Beatty JT, Dobrowolski JM, Bauer CE (1994. a) Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J Mol Biol 237 622–640 [DOI] [PubMed] [Google Scholar]
- Bollivar DW, Wang S, Allen JP, Bauer CE (1994. b) Molecular genetic analysis of terminal steps in bacteriochlorophyll a biosynthesis: characterization of a Rhodobacter capsulatus strain that synthesizes geranylgeraniol-esterified bacteriochlorophyll a. Biochemistry 33 12763–12768 [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Gantt JE (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49 557–583 [DOI] [PubMed] [Google Scholar]
- Dijkwel PP, Huijser C, Weisbeek PJ, Chua NH, Smeekens SCM (1997) Sucrose control of phytochrome A signaling in Arabidopsis. Plant Cell 9 583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichacker LA, Paulsen H, Rüdiger W (1992) Synthesis of chlorophyll a regulates translation of chlorophyll a apoproteins P700, CP47, CP43 and D2 in barley etioplasts. Eur J Biochem 205 17–24 [DOI] [PubMed] [Google Scholar]
- Eichacker LA, Soll J, Lauterbach P, Rüdiger W, Klein RR, Mullet JE (1990) In vitro synthesis of chlorophyll a in the dark triggers accumulation of chlorophyll a apoproteins in barley etioplasts. J Biol Chem 265 13566–13571 [PubMed] [Google Scholar]
- El-Saht HM (2000) Effects of delta-aminolevulinic acid on pigment formation and chlorophyllase activity in French bean leaf. Acta Biol Hung 51 83–90 [PubMed] [Google Scholar]
- Falbel TG, Meehl JB, Staehelin A (1996) Severity of mutant phenotype in a series of chlorophyll-deficient wheat mutants depends on light intensity and the severity of the block in chlorophyll synthesis. Plant Physiol 112 821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falbel TG, Staehelin LA (1996) Partial block in the early steps of the chlorophyll synthesis pathway: a common feature of chlorophyll b-deficient mutants. Plant Physiol 97 311–320 [Google Scholar]
- Flores S, Tobin EM (1986) Cytokinin modulation of Lhcp messenger-RNA levels—the involvement of post-transcriptional regulation. Plant Mol Biol 11 409–415 [DOI] [PubMed] [Google Scholar]
- Fromme P, Melkozernov A, Jordan P, Krauss N (2003) Structure and function of photosystem I: interaction with its soluble electron carriers and external antenna systems. FEBS Lett 555 40–44 [DOI] [PubMed] [Google Scholar]
- Gaubier P, Hui-Ju W, Laudie M, Delseny M, Grellet F (1995) A chlorophyll synthetase gene from Arabidopsis thaliana. Mol Gen Genet 249 58–64 [DOI] [PubMed] [Google Scholar]
- Harper AL, von Gesjen SE, Linford A, Peterson MP, Faircloth RS, Thissen MM, Brusslan JA (2004) Chlorophyllide a oxygenase mRNA and protein levels correlate with the chlorophyll a/b ratio in Arabidopsis thaliana. Photosynth Res 79 149–159 [DOI] [PubMed] [Google Scholar]
- Havaux M, Tardy F (1997) Thermostability and photostability of photosystem II in leaves of the Chlorina-f2 barley mutant deficient in light-harvesting chlorophyll a/b protein complexes. Plant Physiol 113 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6 271–282 [DOI] [PubMed] [Google Scholar]
- Hirai A, Ishibashi T, Morikami A, Iwatsuki N, Shinozaki K, Sugiura M (1985) Rice chloroplast DNA: an oxylase and the 32 kD photosystem II reaction center protein. Theor Appl Genet 70 117–122 [DOI] [PubMed] [Google Scholar]
- International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436 793–800 [DOI] [PubMed] [Google Scholar]
- Jansson S (1994) The light-harvesting chlorophyll a/b-binding proteins. Biochim Biophys Acta 1184 1–19 [DOI] [PubMed] [Google Scholar]
- Jensen PE, Willows RD, Petersen BL, Vothknecht UC, Stummann BM, Kannangara CG, von Wettstein D, Henningsen KW (1996) Structural genes for Mg-chelatase subunits in barley: Xantha-f, -g and -h. Mol Gen Genet 250 383–394 [DOI] [PubMed] [Google Scholar]
- Kaneko T, Tanaka A, Sato S, Kotni H, Sazuka T (1995) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. I. Sequence features in the 1Mb region from map positions 64% to 92% of the genome. DNA Res 2 153–166 [DOI] [PubMed] [Google Scholar]
- Karlin-Neumann GA, Sun L, Tobin EM (1988) Expression of light-harvesting chlorophyll a/b-protein genes is phytochrome regulated in etiolated Arabidopsis thaliana seedlings. Plant Physiol 88 1323–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killough DT, Horlacher WR (1993) The inheritance of virescent yellow and red plant colors in cotton. Genetics 18 329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyozuka J, McElroy D, Hayakawa T, Xie Y, Wu R, Shimamoto K (1993) Light-regulated and cell-specific expression of tomato rbcS-gusA and rice rbcS-gusA fusion genes in transgenic rice. Plant Physiol 102 991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qian Q, Zhou Y, Yan M, Sun L, Zhang M, Fu Z, Wang Y, Han B, Pang X (2003) BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell 15 2020–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2-CT method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Lopez JC, Ryan S, Blankenship RE (1996) Sequence of the bchG gene from Chloroflexus aurantiacus: relationship between chlorophyll synthase and other polyprenyl transferases. J Bacteriol 178 3363–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Fusada N, Osawa N (2003. a) Functional analysis of isoforms of NADPH: protochlorophyllide oxidoreductase (POR), PORB and PORC, in Arabidopsis thaliana. Plant Cell Physiol 44 963–974 [DOI] [PubMed] [Google Scholar]
- Masuda T, Polle JEW, Melis A (2002) Biosynthesis and distribution of chlorophyll among the photosystems during recovery of the green alga Dunaliella salina from irradiance stress. Plant Physiol 128 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Tanaka A, Melis A (2003. b) Chlorophyll antenna size adjustments by irradiance in Dunaliella salina involve coordinate regulation of chlorophyll a oxygenase (CAO) and Lhcb gene expression. Plant Mol Biol 51 757–771 [DOI] [PubMed] [Google Scholar]
- Matsuoka M (1990) Classification and characterization of cDNA that encodes the light-harvesting chlorophyll a/b binding protein of photosystem II from rice. Plant Cell Physiol 31 519–526 [Google Scholar]
- McCouch SR, Teytelman L, Xu YB (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9 199–207 [DOI] [PubMed] [Google Scholar]
- Millar AJ, Kay S (1996) Integration of circadian and phototransduction pathways in the network controlling CAB gene transcription in Arabidopsis. Proc Natl Acad Sci USA 93 15491–15496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock HP, Grimm B (1997) Reduction of uroporphyrin decarboxylase by antisense RNA expression affects activities of other enzymes involved in tetrapyrrole biosynthesis and leads to light-dependent necrosis. Plant Physiol 113 1101–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N, Tanaka R, Satoh S (2005) Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus Species. Plant Cell 17 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagato Y, Yoshimura A (1998) Report of the committee on gene symbolization, nomenclature and linkage groups. Rice Genet Newsl 15 34–74 [Google Scholar]
- Nakayashiki T, Nishimura K, Inokuchi H (1995) Cloning and sequencing of a previously unidentified gene that is involved in the biosynthesis of heme in Escherichia coli. Gene 153 67–70 [DOI] [PubMed] [Google Scholar]
- Narita S, Tanaka R, Ito T, Okada K, Taketani S, Inokuchi H (1996) Molecular cloning and characterization of a cDNA that encodes protoporphyrinogen oxidase of Arabidopsis thaliana. Gene 182 169–175 [DOI] [PubMed] [Google Scholar]
- Nielsen H, Krogh A (1998) Prediction of signal peptides and signal anchors by a hidden Markov model. In Proceedings of the Sixth International Conference on Intelligent Systems for Molecular Biology (ISMB 6). Menlo Park, CA, pp 122–130 [PubMed]
- Nilsson J, Persson B, Heijne GV (2002) Prediction of partial membrane protein topologies using aconsensus approach. Protein Sci 11 2974–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster U, Bauer CE, Rüdiger W (1997) Characterization of chlorophyll a and bacteriochlorophyll a synthases by heterologous expression in Escherichia coli. J Biol Chem 272 9671–9676 [DOI] [PubMed] [Google Scholar]
- Oster U, Rüdiger W (1997) The G4 gene of Arabidopsis thaliana encodes a chlorophyll synthase of etiolated plants. Bot Acta 110 420–423 [Google Scholar]
- Oster U, Tanaka R, Tanaka A, Rüdiger W (2000) Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J 21 305–310 [DOI] [PubMed] [Google Scholar]
- Papenbrock J, Mock HP, Tanaka R, Kruse E, Grimm B (2000) Role of magnesium chelatase activity in the early steps of the tetrapyrrole biosynthetic pathway. Plant Physiol 122 1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen H, Rumler U, Rudiger W (1990) Reconstitution of chlorophyll pigment-containing complexes from light-harvesting chlorophyll a/b-binding protein overexpressed in Escherichia coli. Planta 181 204–211 [DOI] [PubMed] [Google Scholar]
- Pontoppidan B, Kannangara G (1994) Purification and partial characterization of barley glutamyl-tRNA(Glu) reductase, the enzyme that directs glutamate to chlorophyll biosynthesis. Eur J Biochem 225 529–537 [DOI] [PubMed] [Google Scholar]
- Porra RJ (1997) Recent progress in porphyrin and chlorophyll biosynthesis. Photochem Photobiol 63 492–516 [Google Scholar]
- Richard AT (1975) Biochemical Spectroscopy, Vol 1. Adam Hilger Ltd, London, pp 327–330
- Rüdiger W (1992) Last steps in chlorophyll biosynthesis: esterification and insertion into the membrane. In JH Argyroudi-Akoyunoglou, ed, Regulation of Chloroplast Biogenesis. Plenum Press, New York, pp 183–190
- Rüdiger W (1993) Esterification of chlorophyllide and its implication on thylakoid development. In M Ryberg, C Sundqvist, eds, Pigment-Protein Complexes in Plastids: Synthesis and Assembly. Academic Press, New York, pp 219–240
- Rüdiger W, Benz J, Guthoff C (1980) Detection and partial characterization of activity of chlorophyll synthetase in etioplast membranes. Eur J Biochem 109 193–200 [DOI] [PubMed] [Google Scholar]
- Santiago-Ong M, Green RM, Tingay S (2001) shygrl1 is a mutant affected in multiple aspects of photomorphogenesis. Plant Physiol 126 587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid HC, Oster U, Kögel J (2001) Cloning and characterization of chlorophyll synthase from Avena sativa. Biol Chem 382 903–911 [DOI] [PubMed] [Google Scholar]
- Schoch S (1978) The esterification of chlorophyllide a in greening bean leaves. Z Naturforsch 33 712–714 [Google Scholar]
- Schoch S, Lempert U, Rüdiger W (1977) Über die letzten stufen der chlorophyll biosynthesis: zwischenprodukte zwischen chlorophyllid und phytol-haltigem chlorophyll. Z Pflanzenphysiol 83 427–436 [Google Scholar]
- Scolnik P, Bartley GE (1996) A table of some cloned plant genes involved in isoprenoid biosynthesis. Plant Mol Biol Rep 14 305–319 [Google Scholar]
- Soll J, Schultz G (1981) Phytol synthesis from geranylgeraniol in spinach chloroplasts. Biochem Biophys Res Commun 99 907–912 [DOI] [PubMed] [Google Scholar]
- Soll J, Schultz G, Rudiger W, Benz J (1983) Hydrogenation of geranylgeraniol: two pathways exits in spinach chloroplasts. Plant Physiol 71 849–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki JY, Bollivar DW, Bauer CE (1997) Genetic analysis of chlorophyll biosynthesis. Annu Rev Genet 31 61–89 [DOI] [PubMed] [Google Scholar]
- Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K (1998) Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci USA 95 12719–12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Koshino Y, Sawa S, Ishiguro S, Okada K, Tanaka A (2001) Overexpression of chlorophyllide a oxygenase (CAO) enlarges the antenna size of photosystem II in Arabidopsis thaliana. Plant J 26 365–373 [DOI] [PubMed] [Google Scholar]
- Tatsuru M, Naok F, Naoki O (2003) Functional analysis of isoforms of NADPH: protochlorophyllide oxidoreductase (POR), PORB and PORC, in Arabidopsis thaliana. Plant Cell Physiol 44 963–974 [DOI] [PubMed] [Google Scholar]
- Temnykh S, Park W, Ayres N, Cartinhour S, Hauck N, Lipovich L, Cho Y, Ishii T, McCouch S (2000) Mapping and genome organization of microsatellite sequences in rice (Oryza sativa L.). Theor Appl Genet 100 697–712 [Google Scholar]
- Thiel T, Kota R, Grosse I, Stein N, Graner A (2004) SNP2CAPS: a SNP and INDEL analysis tool for CAPS marker development. Nucleic Acids Res 32 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth GJ, Redinbaugh MG, Scandalios JG (1988) A procedure for the small-scale isolation of plant RNA suitable for RNA blot analysis. Anal Biochem 172 279–283 [DOI] [PubMed] [Google Scholar]
- Wanner LA, Gruissem W (1991) Expression dynamics of the tomato rbcS gene family during development. Plant Cell 3 1289–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willows RD (2003) Biosynthesis of chlorophylls from protoporphyrin IX. Nat Prod Rep 20 327–341 [DOI] [PubMed] [Google Scholar]
- Xiong J, Inoue K, Bauer CE (1998) Tracking molecular evolution of photosynthesis by characterization of a major photosynthesis gene cluster from Heliobacillus mobilis. Proc Natl Acad Sci USA 95 14851–14856 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.