Abstract
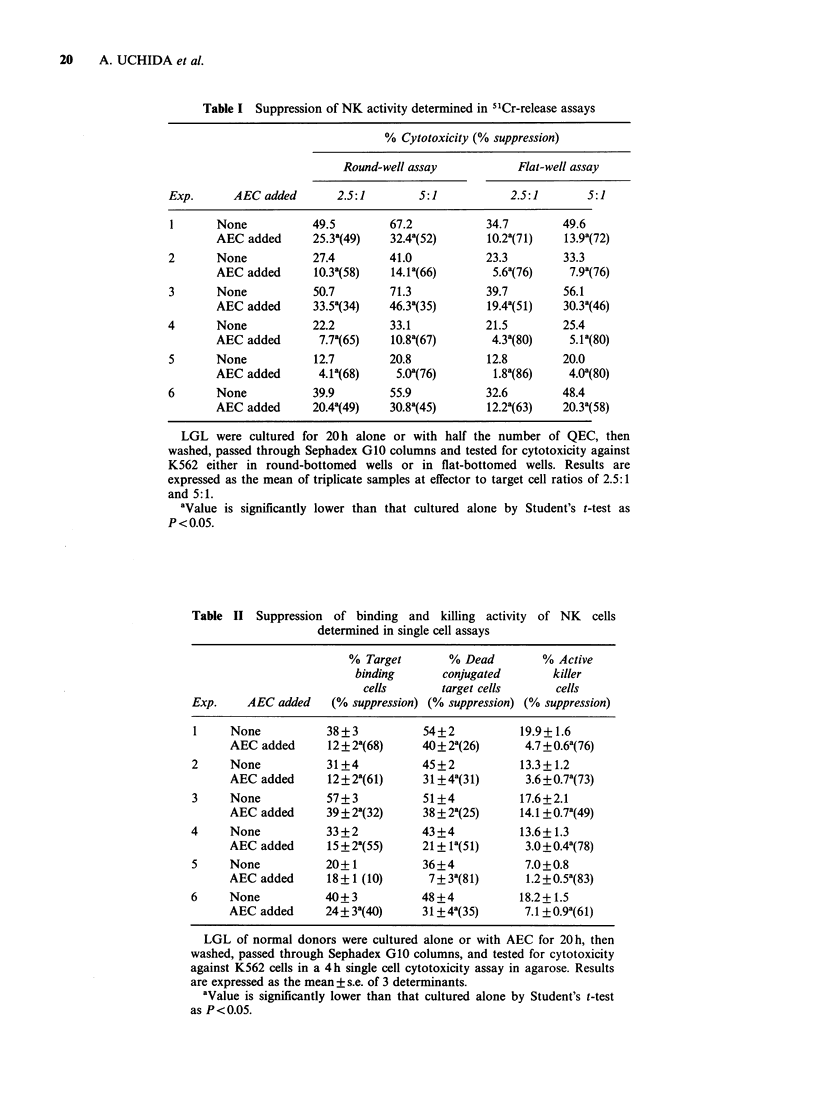
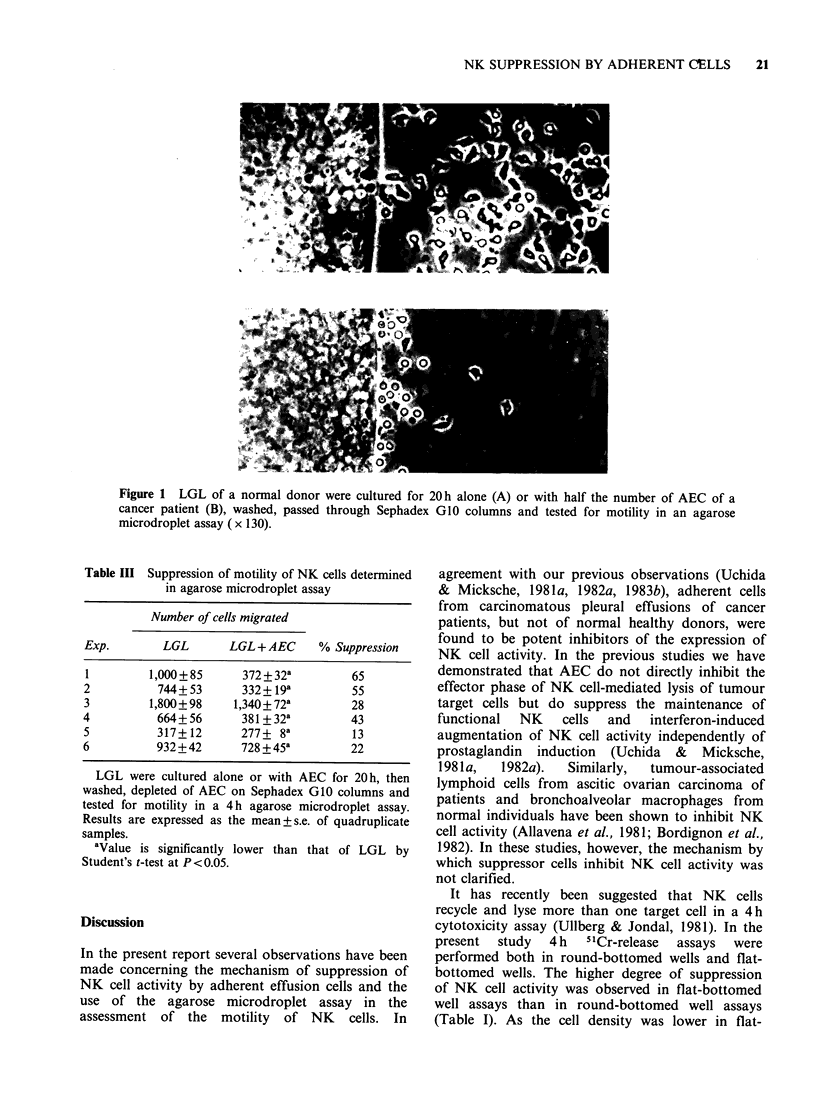
Adherent cells from carcinomatous pleural effusions of lung cancer patients were tested for their ability to suppress natural killer (NK) cell activity, and the mechanism involved in the suppression of NK cell activity was determined. Adherent effusion cells (AEC) were isolated from malignant pleural effusions of patients by centrifugation discontinuous Ficoll-Hypaque gradients and adherence to serum-coated plastic dishes, and large granular lymphocytes (LGL) were purified from the peripheral blood of normal individuals by centrifugation on discontinuous Percoll gradients and further depletion of high-affinity sheep erythrocyte rosette formation. LGL-mediated lysis of K562 cells was suppressed when LGL were cultured with AEC for 20 h, then washed and tested in a 4-h 51Cr release assay. More profound suppression of NK cell activity was observed when cytotoxicity was assayed in flat-bottomed wells rather than in round-bottomed wells. Cytotoxicity assays conducted at the single cell level in agarose revealed that the frequency of LGL binding to K562 cells and of dead conjugated target cells was reduced after overnight contact with AEC. In agarose microdroplet assays, functional LGL from normal donors exhibited definitive motility, expressing polarized shape. In contrast, a small number of LGL with non-polarized configuration migrated from the agarose droplet after overnight culture with AEC. These results indicate that functionally suppressed NK cells lose their motility, binding capacity and killing activity, which could be responsible for the suppression of NK cell activity by AEC.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allavena P., Introna M., Mangioni C., Mantovani A. Inhibition of natural killer activity by tumor-associated lymphoid cells from ascites ovarian carcinomas. J Natl Cancer Inst. 1981 Aug;67(2):319–325. [PubMed] [Google Scholar]
- Bordignon C., Villa F., Allavena P., Introna M., Biondi A., Avallone R., Mantovani A. Inhibition of natural killer activity by human bronchoalveolar macrophages. J Immunol. 1982 Aug;129(2):587–591. [PubMed] [Google Scholar]
- Carpén O., Virtanen I., Saksela E. The cytotoxic activity of human natural killer cells requires an intact secretory apparatus. Cell Immunol. 1981 Feb;58(1):97–106. doi: 10.1016/0008-8749(81)90152-0. [DOI] [PubMed] [Google Scholar]
- Cudkowicz G., Hochman P. S. Do natural killer cells engage in regulated reactions against self to ensure homeostasis? Immunol Rev. 1979;44:13–41. doi: 10.1111/j.1600-065x.1979.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Gerson J., Varesio L., Herberman R. B. Systemic and in situ natural killer and suppressor cell activities in mice bearing progressively growing murine sarcoma-virus-induced tumors. Int J Cancer. 1981 Feb 15;27(2):243–248. doi: 10.1002/ijc.2910270218. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Grimm E., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. I. Estimation of cytotoxic T lymphocyte frequency and relative lytic efficiency. J Immunol. 1979 Dec;123(6):2861–2869. [PubMed] [Google Scholar]
- Hiserodt J. C., Britvan L. J., Targan S. R. Characterization of the cytolytic reaction mechanism of the human natural killer (NK) lymphocyte: resolution into binding, programming, and killer cell-independent steps. J Immunol. 1982 Oct;129(4):1782–1787. [PubMed] [Google Scholar]
- Kendall R. A., Targan S. The dual effect of prostaglandin (PGE2) and ethanol on the natural killer cytolytic process: effector activation and NK-cell-target cell conjugate lytic inhibition. J Immunol. 1980 Dec;125(6):2770–2777. [PubMed] [Google Scholar]
- Santoni A., Riccardi C., Barlozzari T., Herberman R. B. Suppression of activity of mouse natural killer (NK) cells by activated macrophages from mice treated with pyran copolymer. Int J Cancer. 1980 Dec 15;26(6):837–843. doi: 10.1002/ijc.2910260619. [DOI] [PubMed] [Google Scholar]
- Savary C. A., Lotzová E. Suppression of natural killer cell cytotoxicity by splenocytes from Corynebacterium parvum-injected, bone marrow-tolerant, and infant mice. J Immunol. 1978 Jan;120(1):239–243. [PubMed] [Google Scholar]
- Tagliabue A., Herberman R. B., McCoy J. L. Cellular immunity to mammary tumor virus in normal and tumor-bearing C3H/HeN mice. Cancer Res. 1978 Aug;38(8):2279–2284. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Analysis by a single cell cytotoxicity assay of natural killer (NK) cells frequencies among human large granular lymphocytes and of the effects of interferon on their activity. J Immunol. 1982 Jun;128(6):2514–2521. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A., Kolb R., Micksche M. Generation of suppressor cells for natural killer activity in cancer patients after surgery. J Natl Cancer Inst. 1982 May;68(5):735–741. [PubMed] [Google Scholar]
- Uchida A., Micksche M. Intrapleural administration of OK432 in cancer patients: activation of NK cells and reduction of suppressor cells. Int J Cancer. 1983 Jan 15;31(1):1–5. doi: 10.1002/ijc.2910310102. [DOI] [PubMed] [Google Scholar]
- Uchida A., Micksche M. Lysis of fresh human tumor cells by autologous large granular lymphocytes from peripheral blood and pleural effusions. Int J Cancer. 1983 Jul 15;32(1):37–44. doi: 10.1002/ijc.2910320107. [DOI] [PubMed] [Google Scholar]
- Ullberg M., Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981 Mar 1;153(3):615–628. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]



