Abstract
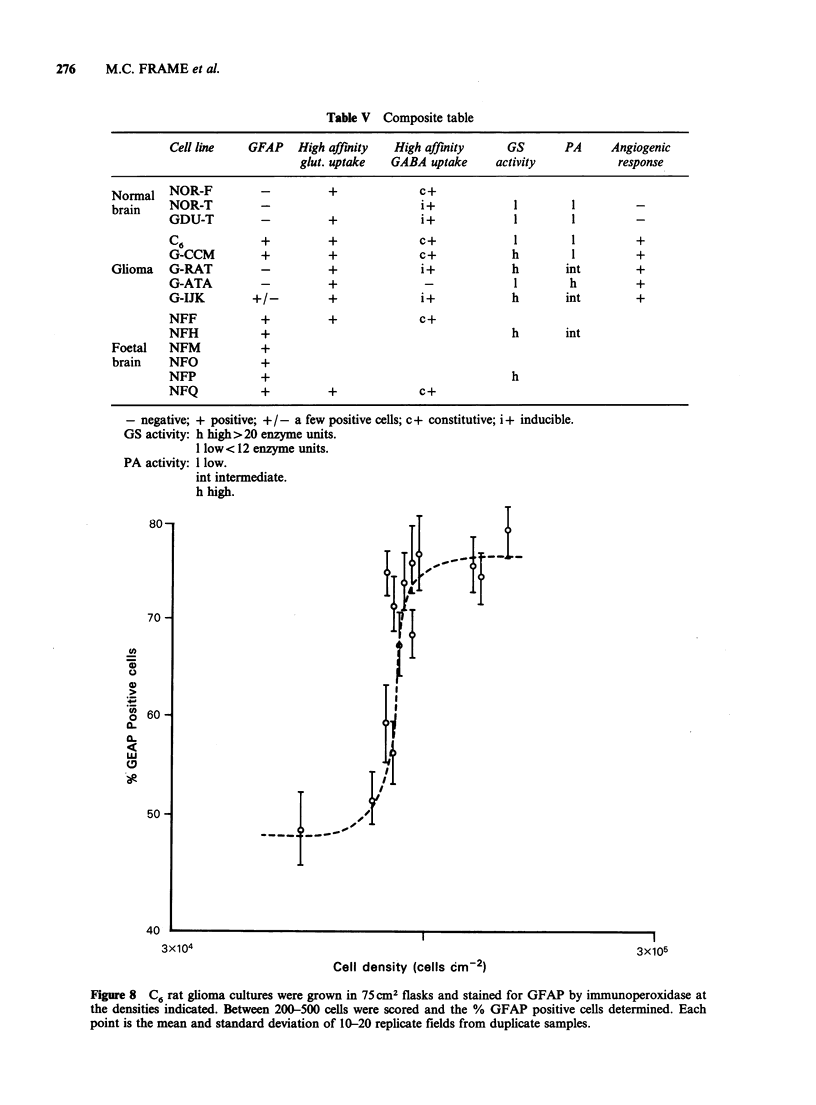
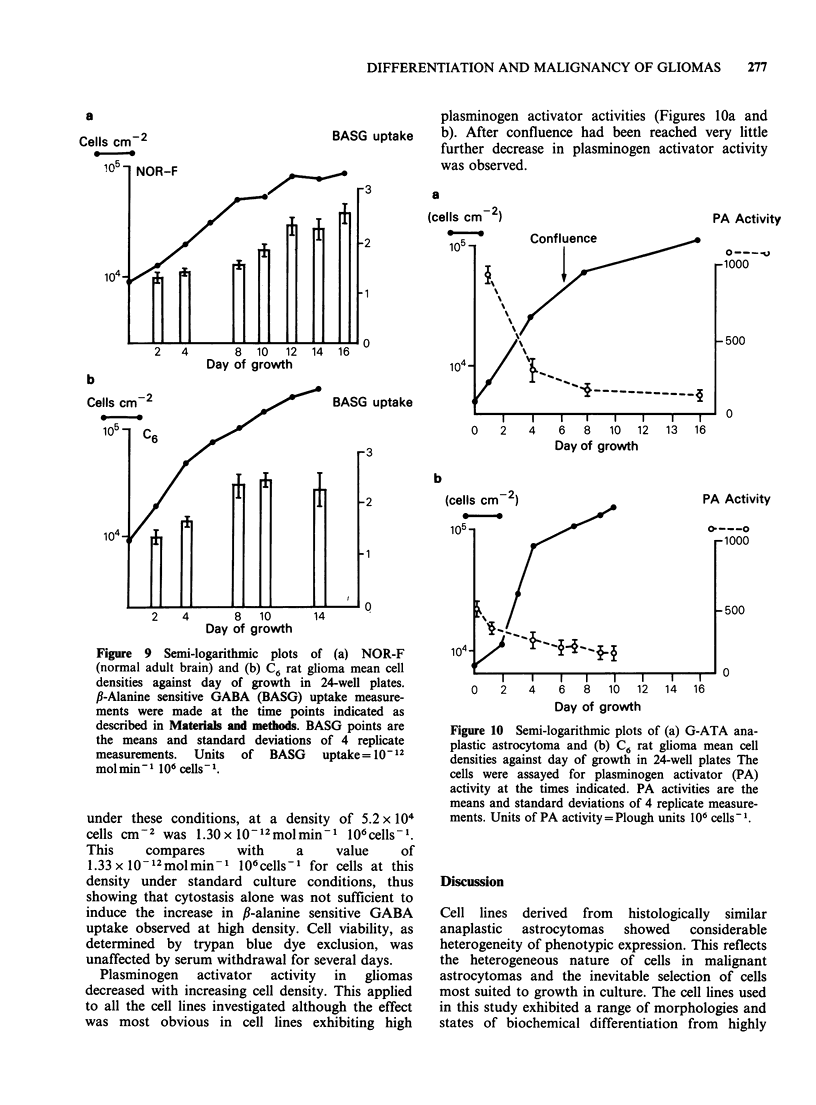
The phenotypic expression of cells derived from human anaplastic astrocytomas, rat glioma, normal human adult and foetal brain tissue have been examined for differentiated and malignancy-associated properties. Glial fibrillary acidic protein (GFAP), high affinity glutamate and gamma-amino butyric acid (GABA) uptake and glutamine synthetase were used as indicators of astroglial differentiation. Plasminogen activator and tumour angiogenesis factor were the malignancy-associated markers. The normal adult brain-derived lines showed some differentiated astroglial features and expressed low levels of the malignancy-associated properties. The foetal cultures contained highly differentiated astroglia while the glioma lines showed considerable phenotypic heterogeneity from highly differentiated to undifferentiated. The least differentiated glioma cells exhibited the highest plasminogen activator activities. The density-dependent control of phenotypic expression was also investigated. High affinity GABA uptake, and GFAP in rat C6 glioma cultures, increased with increasing monolayer cell density, events probably mediated by an increase in the formation of cell-cell contacts at confluence. Plasminogen activator activity decreased with increasing cell density.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett K., McGinnis J. F., de Vellis J. Reversible inhibition of the hydrocortisone induction of glycerol phosphate dehydrogenase by cytochalasin B in rat glial C6 cells. J Cell Physiol. 1977 Nov;93(2):247–260. doi: 10.1002/jcp.1040930210. [DOI] [PubMed] [Google Scholar]
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Rubinstein L. J. Contribution of immunohistochemistry to diagnostic problems of human cerebral tumors. J Histochem Cytochem. 1978 Jul;26(7):513–522. doi: 10.1177/26.7.357640. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- FEIGIN I., ALLEN L. B., LIPKIN L., GROSS S. W. The endothelial hyperplasia of the cerebral blood vessels with brain tumors, and its sarcomatous transformation. Cancer. 1958 Mar-Apr;11(2):264–277. doi: 10.1002/1097-0142(195803/04)11:2<264::aid-cncr2820110207>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Guner M., Freshney R. I., Morgan D., Freshney M. G., Thomas D. G., Graham D. I. Effects of dexamethasone and betamethasone on in vitro cultures from human astrocytoma. Br J Cancer. 1977 Apr;35(4):439–447. doi: 10.1038/bjc.1977.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallermayer K., Harmening C., Hamprecht B. Cellular localization and regulation of glutamine synthetase in primary cultures of brain cells from newborn mice. J Neurochem. 1981 Jul;37(1):43–52. doi: 10.1111/j.1471-4159.1981.tb05289.x. [DOI] [PubMed] [Google Scholar]
- Hince T. A., Roscoe J. P. Fibrinolytic activity of cultured cells derived during ethylnitrosourea-induced carcinogenesis of rat brain. Br J Cancer. 1978 Mar;37(3):424–433. doi: 10.1038/bjc.1978.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Krylova N. V. Endothelium characteristics of blood vessels in neoplasms. Bibl Anat. 1973;12:497–503. [PubMed] [Google Scholar]
- Logan W. J., Snyder S. H. Unique high affinity uptake systems for glycine, glutamic and aspartic acids in central nervous tissue of the rat. Nature. 1971 Dec 3;234(5327):297–299. doi: 10.1038/234297b0. [DOI] [PubMed] [Google Scholar]
- Mak T. W., Rutledge G., Sutherland D. J. Androgen-dependent fibrinolytic activity in a murine mammary carcinoma (Shionogi SC-115 cells) in vitro. Cell. 1976 Feb;7(2):223–226. doi: 10.1016/0092-8674(76)90021-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Bell K. P., Norenberg M. D. Glutamine synthetase: glial localization in brain. Science. 1977 Mar 25;195(4284):1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Palfreyman J. W., Thomas D. G., Ratcliffe J. G., Graham D. I. Glial fibrillary acidic protein (GFAP): purification from human fibrillary astrocytoma, development and validation of a radioimmunoassay for GFAP-like immunoactivity. J Neurol Sci. 1979 Mar;41(1):101–113. doi: 10.1016/0022-510x(79)90144-8. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Hynes R. O., Franks L. M., Hemmings V. J. Surface proteins and fibrinolytic activity of cultured mammalian cells. Cancer Res. 1976 Apr;36(4):1475–1480. [PubMed] [Google Scholar]
- Peña C. E., Felter R. Ultrastructure of a composite glioma-sarcoma of the brain. Acta Neuropathol. 1973;23(1):90–94. doi: 10.1007/BF00689008. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S. E., Herschman H. R., Lightbody J., Sato G. Synthesis by a clonal line of rat glial cells of a protein unique to the nervous system. J Cell Physiol. 1970 Jun;75(3):329–339. doi: 10.1002/jcp.1040750309. [DOI] [PubMed] [Google Scholar]
- Pollack R., Risser R., Conlon S., Rifkin D. Plasminogen activator production accompanies loss of anchorage regulation in transformation of primary rat embryo cells by simian virus 40. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4792–4796. doi: 10.1073/pnas.71.12.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif-Lehrer L. Actinomycin-D enhancement of glutamine synthetase activity in chick embryo retinas cultured in the presence of cortisol. J Cell Biol. 1971 Oct;51(1):303–311. doi: 10.1083/jcb.51.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Loeb J. N., Moore G., Reich E. Properties of plasminogen activators formed by neoplastic human cell cultures. J Exp Med. 1974 May 1;139(5):1317–1328. doi: 10.1084/jem.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A., Svenneby G., Hertz L. Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J Neurochem. 1977 Dec;29(6):999–1005. doi: 10.1111/j.1471-4159.1977.tb06503.x. [DOI] [PubMed] [Google Scholar]
- Tucker W. S., Kirsch W. M., Martinez-Hernandez A., Fink L. M. In vitro plasminogen activator activity in human brain tumors. Cancer Res. 1978 Feb;38(2):297–302. [PubMed] [Google Scholar]
- Velasco M. E., Dahl D., Roessmann U., Gambetti P. Immunohistochemical localization of glial fibrillary acidic protein in human glial neoplasms. Cancer. 1980 Feb;45(3):484–494. doi: 10.1002/1097-0142(19800201)45:3<484::aid-cncr2820450312>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Vidard M. N., Girard N., Chauzy C., Delpech B., Delpech A., Maunoury R., Laumonier R. Disparition de la protéine gliofibrillaire (GFA) au cours de la culture de cellules de glioblastomes. C R Acad Sci Hebd Seances Acad Sci D. 1978 Jun 19;286(24):1837–1840. [PubMed] [Google Scholar]
- Wade D. R., Burkart M. E. The role of adenosine 3',5'-cyclic monophosphate in the density-dependent regulation of growth and tyrosinase activity of B-16 melanoma cells. J Cell Physiol. 1978 Mar;94(3):265–273. doi: 10.1002/jcp.1040940304. [DOI] [PubMed] [Google Scholar]
- Westermark B. The deficient density-dependent growth control of human malignant glioma cells and virus-transformed glia-like cells in culture. Int J Cancer. 1973 Sep 15;12(2):438–451. doi: 10.1002/ijc.2910120215. [DOI] [PubMed] [Google Scholar]
- Whur P., Magudia M., Boston J., Lockwood J., Williams D. C. Plasminogen activator in cultured Lewis lung carcinoma cells measured by chromogenic substrate assay. Br J Cancer. 1980 Aug;42(2):305–313. doi: 10.1038/bjc.1980.231. [DOI] [PMC free article] [PubMed] [Google Scholar]