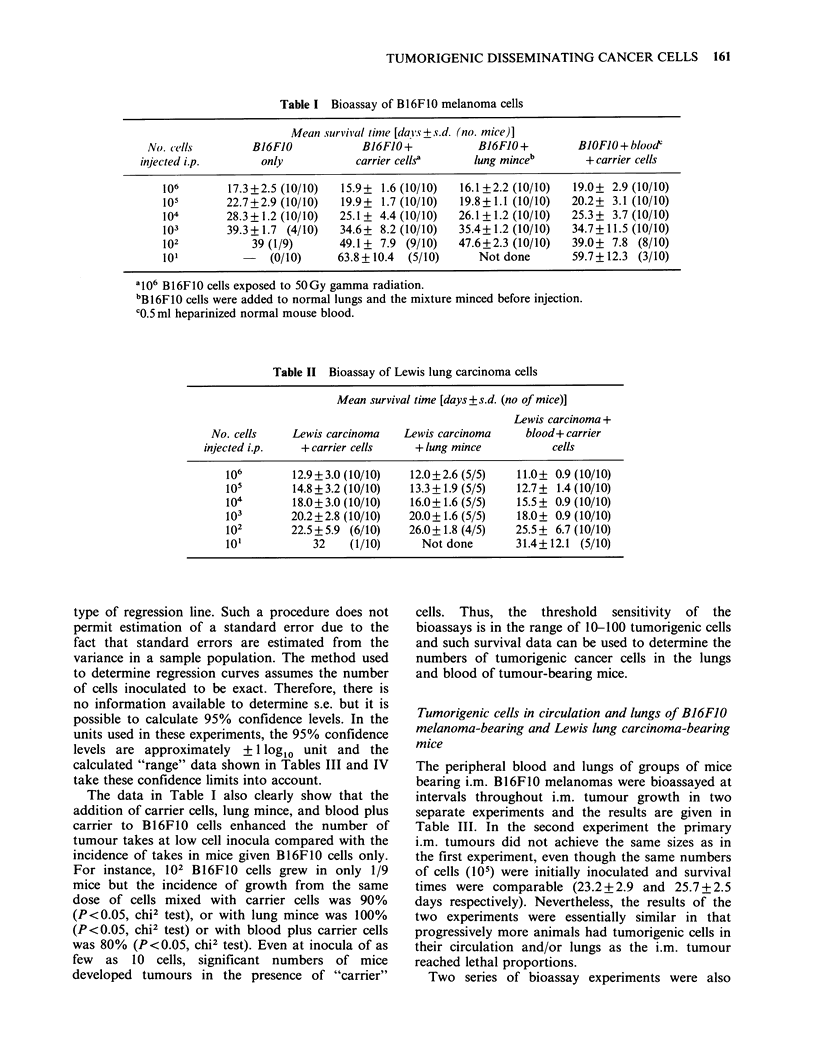
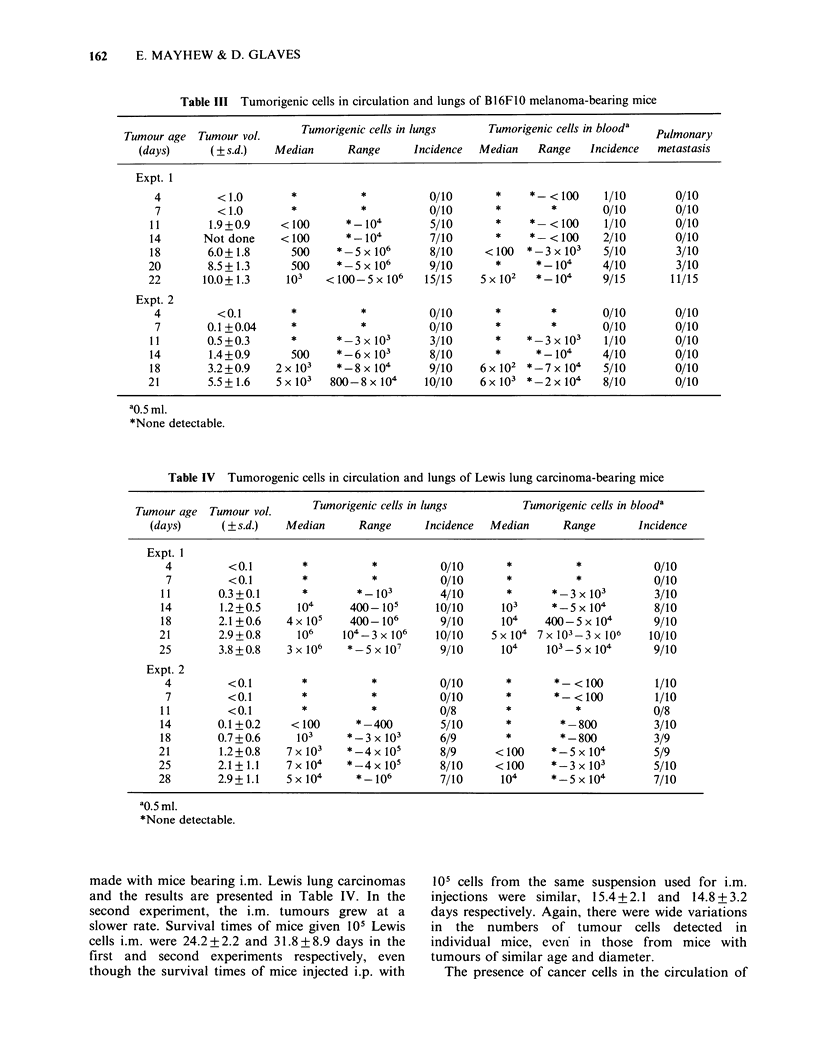
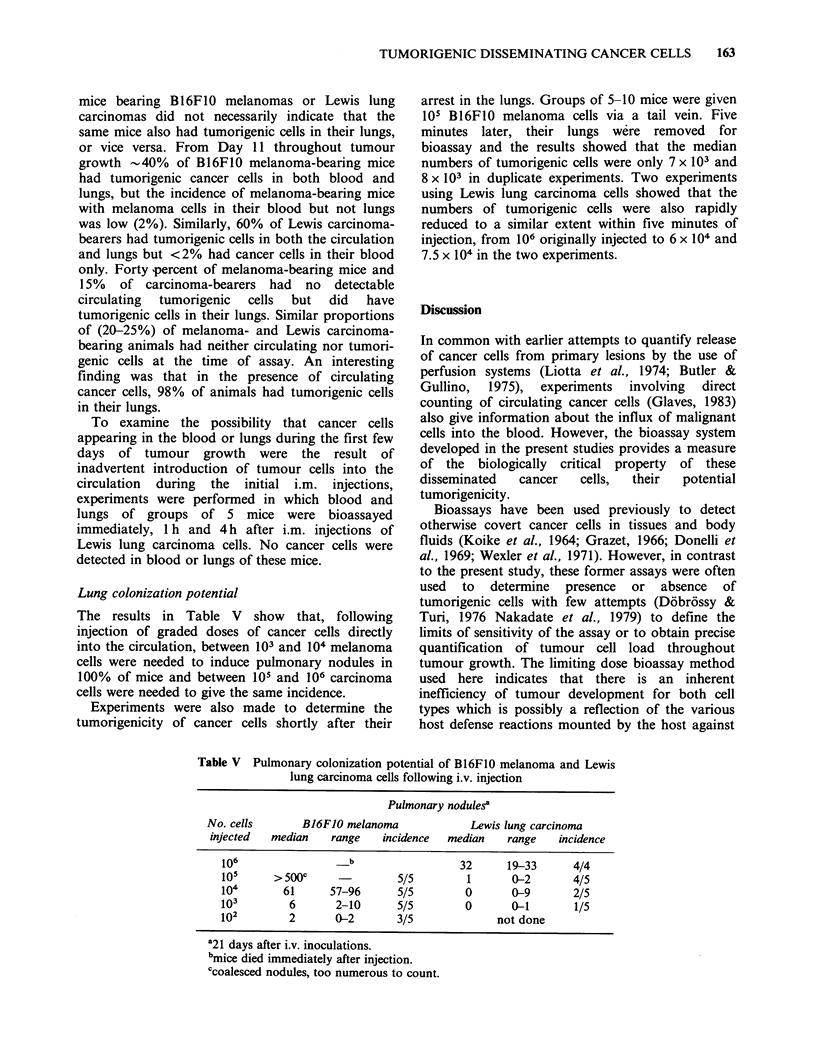
Abstract
The numbers of potentially tumorigenic cancer cells released into the circulation and secondarily arrested in the lungs of mice bearing B16F10 melanomas or Lewis lung carcinomas were systematically quantified throughout i.m. tumour growth using a bioassay procedure capable of detecting as few as 10 to 100 tumorigenic cells in the circulation or lungs. Viable disseminating cancer cells were detectable within 4 days of i.m. tumour growth and reached 10(6) per 0.5 ml of blood in carcinoma-bearers and 2 X 10(4) per 0.5 ml in melanoma-bearers; 98% of mice with circulating cancer cells had potentially tumorigenic cells in their lungs, even in the absence of overt metastases. The numbers of cancer cells present in the circulation and lungs were related to the growth rate of the i.m. lesion, more cells being released from faster-growing tumours. The numbers of tumorigenic carcinoma cells were compared with the total numbers of cells released into the circulation as quantitated by direct counting procedures, and it was found that the vast majority of these circulating cells were potentially tumorigenic. These studies provide quantitative information about cancer cell input into the metastatic process. Also, the bioassay procedure provides a useful experimental model for the development of regimens for therapy of metastases since it is a sensitive method of monitoring not only the size of disseminated populations of cancer cells but also their clinically relevant property namely, their tumorigenic potential.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler T. P., Gullino P. M. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975 Mar;35(3):512–516. [PubMed] [Google Scholar]
- Donelli M. G., Rosso R., Garattini S. Quantitative studies on cancer dissemination. Cancer Res. 1969 Feb;29(2):414–418. [PubMed] [Google Scholar]
- Döbrössy L., Túri G. Bioassay of blood-born tumour cells on in vivo and in vitro systems. Acta Morphol Acad Sci Hung. 1976;24(4):331–340. [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Budmen M. B. Characterization in vivo and in vitro of tumor cells selected for resistance to syngeneic lymphocyte-mediated cytotoxicity. Cancer Res. 1976 Sep;36(9 PT1):3160–3165. [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Riggs C. W. Relationship of host immune status to tumor cell arrest, distribution, and survival in experimental metastasis. Cancer. 1977 Jul;40(1):46–55. doi: 10.1002/1097-0142(197707)40:1<46::aid-cncr2820400110>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer. 1983 Nov;48(5):665–673. doi: 10.1038/bjc.1983.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaves D. Metastasis: reticuloendothelial system and organ retention of disseminated malignant cells. Int J Cancer. 1980 Jul 15;26(1):115–122. doi: 10.1002/ijc.2910260118. [DOI] [PubMed] [Google Scholar]
- KOIKE A. MECHANISM OF BLOOD-BORNE METASTASES. I. SOME FACTORS AFFECTING LODGMENT AND GROWTH OF TUMOR CELLS IN THE LUNGS. Cancer. 1964 Apr;17:450–460. doi: 10.1002/1097-0142(196404)17:4<450::aid-cncr2820170406>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Kleinerman J., Saidel G. M. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974 May;34(5):997–1004. [PubMed] [Google Scholar]
- Nakadate T., Suzuki M., Sato H. Quantitative study on the liberation of tumor cells into the circulating blood. Gan. 1979 Aug;70(4):435–446. [PubMed] [Google Scholar]
- REVESZ L. Effect of X-irradiation on the growth of the Ehrlich ascites tumor. J Natl Cancer Inst. 1955 Jun;15(6):1691–1701. [PubMed] [Google Scholar]
- Riccardi C., Puccetti P., Santoni A., Herberman R. B. Rapid in vivo assay of mouse natural killer cell activity. J Natl Cancer Inst. 1979 Oct;63(4):1041–1045. [PubMed] [Google Scholar]
- SKIPPER H. E., SCHABEL F. M., Jr, WILCOX W. S. EXPERIMENTAL EVALUATION OF POTENTIAL ANTICANCER AGENTS. XIII. ON THE CRITERIA AND KINETICS ASSOCIATED WITH "CURABILITY" OF EXPERIMENTAL LEUKEMIA. Cancer Chemother Rep. 1964 Feb;35:1–111. [PubMed] [Google Scholar]
- SUGIURA K., STOCK C. C. Studies in a tumor spectrum. III. The effect of phosphoramides on the growth of a variety of mouse and rat tumors. Cancer Res. 1955 Jan;15(1):38–51. [PubMed] [Google Scholar]
- Schirrmacher V., Waller C. A. Quantitative determination of disseminated tumor cells by [3H]thymidine incorporation in vitro and by agar colony formation. Cancer Res. 1982 Feb;42(2):660–666. [PubMed] [Google Scholar]
- Skipper H. E., Schabel F. M., Jr, Wilcox W. S., Laster W. R., Jr, Trader M. W., Thompson S. A. Experimental evaluation of potential anticancer agents. 28. Effects of therapy on viability and rate of proliferation of leukemic cells in various anatomic sites. Cancer Chemother Rep. 1965 Aug;47:41–64. [PubMed] [Google Scholar]
- WEISS L. STUDIES ON CELLULAR ADHESION IN TISSUE CULTURE. VII. SURFACE ACTIVITY AND CELL DETACHMENT. Exp Cell Res. 1964 Jan;33:277–288. doi: 10.1016/s0014-4827(64)81033-8. [DOI] [PubMed] [Google Scholar]
- Weiss L., Glaves D. The immunospecificity of altered initial arrest patterns of circulating cancer cells in tumor-bearing mice. Int J Cancer. 1976 Dec 15;18(6):774–777. doi: 10.1002/ijc.2910180608. [DOI] [PubMed] [Google Scholar]
- Weiss L., Mayhew E., Rapp D. G., Holmes J. C. Metastatic inefficiency in mice bearing B16 melanomas. Br J Cancer. 1982 Jan;45(1):44–53. doi: 10.1038/bjc.1982.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H., Kenady D. E., Ketcham A. S. Bioassay of circulating murine tumor cells: a comparison between intravenous and intramuscular inoculation on tumor incidence. Cancer. 1971 Jan;27(1):56–60. doi: 10.1002/1097-0142(197101)27:1<56::aid-cncr2820270110>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


