Abstract
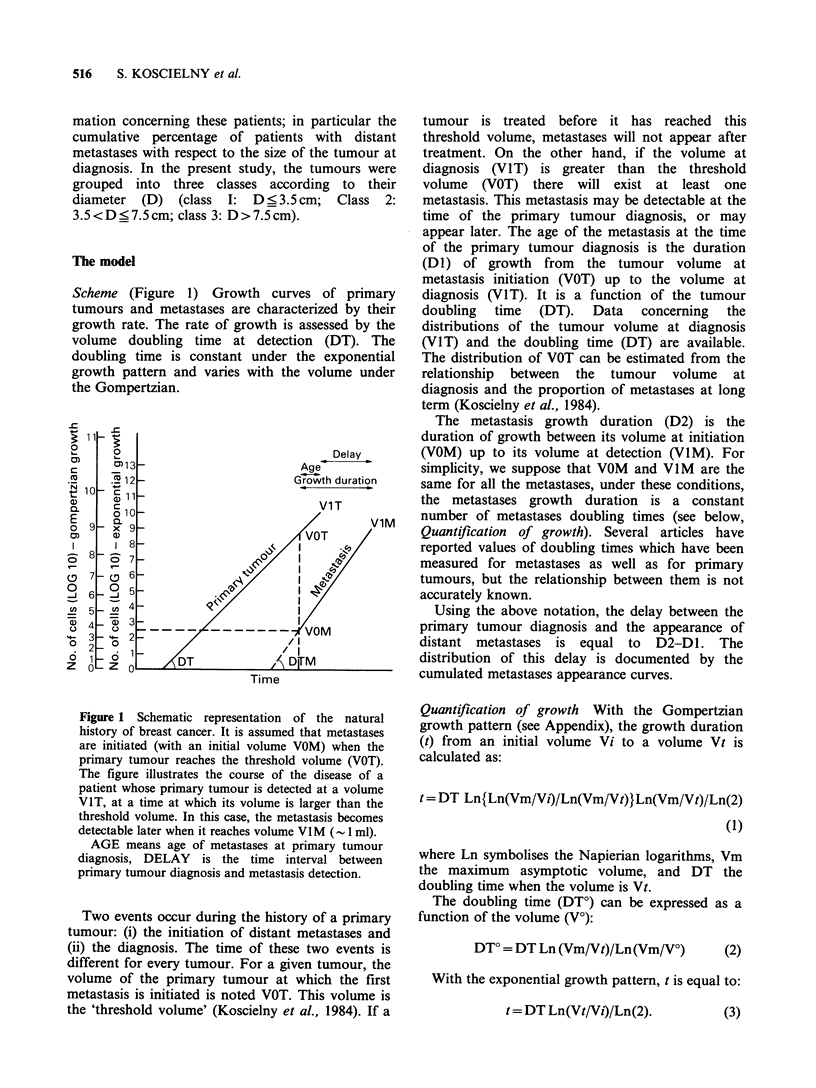
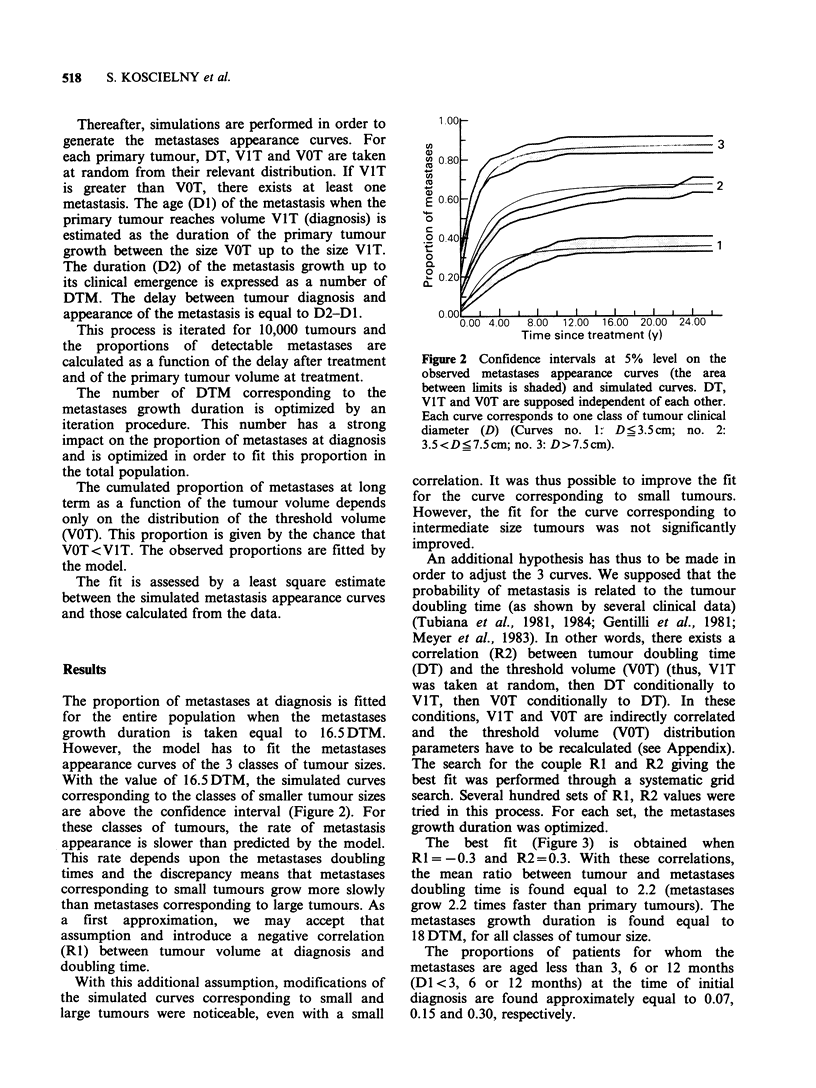
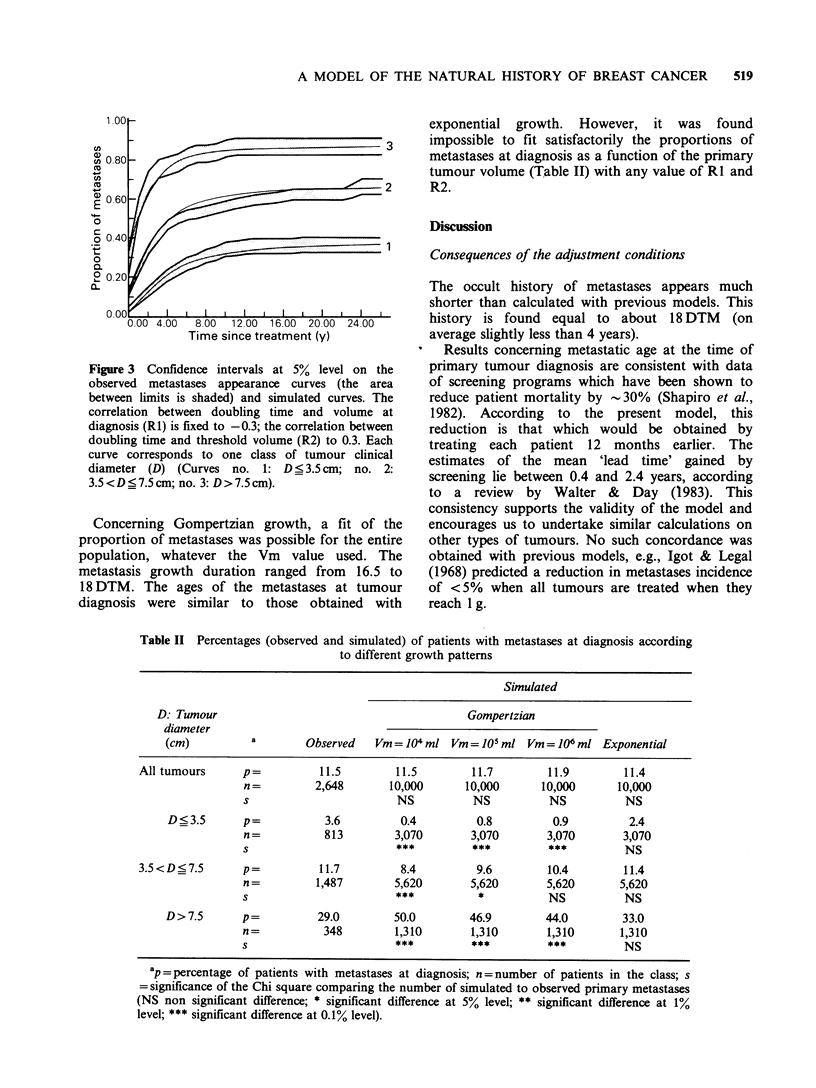
In order to assess the time at which the distant metastases were initiated, a model has been developed to simulate the natural history of human breast cancer. The metastasis appearance curves were fitted to those observed for tumours of various sizes among the 2648 patients treated at the Institut Gustave Roussy from 1954 to 1972. The model assumes that metastases are initiated when the tumour reaches a threshold volume (distribution of this volume was estimated in a previous article). Two patterns of growth were considered: exponential and Gompertzian. Distributions of tumour and metastases doubling times are fixed according to the literature. A relationship between tumour and metastasis doubling time is estimated. Simulations were used to optimize metastases growth duration as a function of the metastasis doubling time. The ages of the metastases at tumour diagnosis are calculated. With exponential growth, it was necessary to introduce correlations to obtain a satisfactory fit of the metastases appearance curves: between the tumour volume at diagnosis and the doubling time (R1 = -0.3), and between the tumour volume at metastasis initiation and the doubling time (R2 = 0.3). The growth duration of the metastases before their detection was found to equal about 18 metastases doubling times at detection and the mean ratio between the doubling time of a tumour and its metastases equal to 2.2. With Gompertzian growth, it was impossible to adjust satisfactorily the proportions of metastases at diagnosis as a function of the primary tumour volume. However, when we ignore this, the best fit was obtained when the duration of metastases growth before detection was about the same as for exponential growth. With either growth pattern, the model predicts that the proportion of patients with metastases would be reduced by approximately 30% if the primary tumours were treated 12 months earlier. This prediction is consistent with the results of the screening programs for breast cancer.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P. Do cancers arise from a single transformed cell or is monoclonality of tumours a late event in carcinogenesis? Br J Cancer. 1985 Apr;51(4):453–457. doi: 10.1038/bjc.1985.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Malaise E. P., Tubiana M. Relation between the pathological nature and the growth rate of human tumors. Eur J Cancer. 1971 Aug;7(4):307–315. doi: 10.1016/0014-2964(71)90073-9. [DOI] [PubMed] [Google Scholar]
- Combes P. F., Douchez J., Carton M., Naja A. Etude de la croissance des métastases pulmonaires humaines comme argument objectif d'évaluation du pronostic et des effets thérapeutiques (basée sur 90 observations) J Radiol Electrol Med Nucl. 1968 Dec;49(12):893–901. [PubMed] [Google Scholar]
- Edwards M. H., Baum M., Magarey C. J. Regression of axillary lymph-nodes in cancer of the breast. Br J Surg. 1972 Oct;59(10):776–779. doi: 10.1002/bjs.1800591008. [DOI] [PubMed] [Google Scholar]
- GERSHON-COHEN J., BERGER S. M., KLICKSTEIN H. S. ROENTGENOGRAPHY OF BREAST CANCER MODERATING CONCEPT OF "BIOLOGIC PREDETERMINISM". Cancer. 1963 Aug;16:961–964. doi: 10.1002/1097-0142(196308)16:8<961::aid-cncr2820160802>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gentili C., Sanfilippo O., Silvestrini R. Cell proliferation and its relationship to clinical features and relapse in breast cancers. Cancer. 1981 Aug 15;48(4):974–979. doi: 10.1002/1097-0142(19810815)48:4<974::aid-cncr2820480420>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Heuser L., Spratt J. S., Jr, Polk H. C., Jr, Buchanan J. Relation between mammary cancer growth kinetics and the intervals between screenings. Cancer. 1979 Mar;43(3):857–862. doi: 10.1002/1097-0142(197903)43:3<857::aid-cncr2820430312>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Igot J. P., Le Gal Y. Age des adénopathies métastatiques dans le cancer mammaire. Ann Anat Pathol (Paris) 1968 Oct-Dec;13(4):449–459. [PubMed] [Google Scholar]
- Koscielny S., Tubiana M., Lê M. G., Valleron A. J., Mouriesse H., Contesso G., Sarrazin D. Breast cancer: relationship between the size of the primary tumour and the probability of metastatic dissemination. Br J Cancer. 1984 Jun;49(6):709–715. doi: 10.1038/bjc.1984.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama S., Spratt J. S., Jr, Donegan W. L., Watson F. R., Cunningham C. The cross rates of growth of human mammary carcinoma. Cancer. 1972 Aug;30(2):594–599. doi: 10.1002/1097-0142(197208)30:2<594::aid-cncr2820300241>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Lundgren B. Observations on growth rate of breast carcinomas and its possible implications for lead time. Cancer. 1977 Oct;40(4):1722–1725. doi: 10.1002/1097-0142(197710)40:4<1722::aid-cncr2820400448>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mackillop W. J., Ciampi A., Till J. E., Buick R. N. A stem cell model of human tumor growth: implications for tumor cell clonogenic assays. J Natl Cancer Inst. 1983 Jan;70(1):9–16. [PubMed] [Google Scholar]
- Malaise E. P., Chavaudra N., Charbit A., Tubiana M. Relationship between the growth rate of human metastases, survival and pathological type. Eur J Cancer. 1974 Jul;10(7):451–459. doi: 10.1016/0014-2964(74)90029-2. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Friedman E., McCrate M. M., Bauer W. C. Prediction of early course of breast carcinoma by thymidine labeling. Cancer. 1983 May 15;51(10):1879–1886. doi: 10.1002/1097-0142(19830515)51:10<1879::aid-cncr2820511021>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Pearlman A. W. Breast cancer--influence of growth rate on prognosis and treatment evaluation: a study based on mastectomy scar recurrences. Cancer. 1976 Oct;38(4):1826–1833. doi: 10.1002/1097-0142(197610)38:4<1826::aid-cncr2820380460>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Philippe E., Le Gal Y. Growth of seventy-eight recurrent mammary cancers. Quantitative study. Cancer. 1968 Mar;21(3):461–467. doi: 10.1002/1097-0142(196803)21:3<461::aid-cncr2820210317>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M. A biomathematical approach to clinical tumor growth. Cancer. 1961 Nov-Dec;14:1272–1294. doi: 10.1002/1097-0142(196111/12)14:6<1272::aid-cncr2820140618>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- SPRATT J. S., Jr, SPRATT T. L. RATES OF GROWTH OF PULMONARY METASTASES AND HOST SURVIVAL. Ann Surg. 1964 Feb;159:161–171. doi: 10.1097/00000658-196402000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S., Venet W., Strax P., Venet L., Roeser R. Ten- to fourteen-year effect of screening on breast cancer mortality. J Natl Cancer Inst. 1982 Aug;69(2):349–355. [PubMed] [Google Scholar]
- Slack N. H., Blumenson L. E., Bross I. D. Therapeutic implications from a mathematical model characterizing the course of breast cancer. Cancer. 1969 Nov;24(5):960–971. doi: 10.1002/1097-0142(196911)24:5<960::aid-cncr2820240515>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Trott K. R., Maciejewski B., Preuss-Bayer G., Skolyszewski J. Dose-response curve and split-dose recovery in human skin cancer. Radiother Oncol. 1984 Aug;2(2):123–129. doi: 10.1016/s0167-8140(84)80048-1. [DOI] [PubMed] [Google Scholar]
- Tubiana M., Chauvel P., Renaud A., Malaise E. P. Vitesse de croissance et histoire naturelle du cancer du sein. Bull Cancer. 1975 Oct-Dec;62(4):341–358. [PubMed] [Google Scholar]
- Tubiana M. L.H. Gray Medal lecture: cell kinetics and radiation oncology. Int J Radiat Oncol Biol Phys. 1982 Sep;8(9):1471–1489. doi: 10.1016/0360-3016(82)90607-1. [DOI] [PubMed] [Google Scholar]
- Tubiana M., Pejovic M. H., Chavaudra N., Contesso G., Malaise E. P. The long-term prognostic significance of the thymidine labelling index in breast cancer. Int J Cancer. 1984 Apr 15;33(4):441–445. doi: 10.1002/ijc.2910330404. [DOI] [PubMed] [Google Scholar]
- Tubiana M., Pejovic M. J., Renaud A., Contesso G., Chavaudra N., Gioanni J., Malaise E. P. Kinetic parameters and the course of the disease in breast cancer. Cancer. 1981 Mar 1;47(5):937–943. doi: 10.1002/1097-0142(19810301)47:5<937::aid-cncr2820470520>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Walter S. D., Day N. E. Estimation of the duration of a pre-clinical disease state using screening data. Am J Epidemiol. 1983 Dec;118(6):865–886. doi: 10.1093/oxfordjournals.aje.a113705. [DOI] [PubMed] [Google Scholar]
- von Fournier D., Weber E., Hoeffken W., Bauer M., Kubli F., Barth V. Growth rate of 147 mammary carcinomas. Cancer. 1980 Apr 15;45(8):2198–2207. doi: 10.1002/1097-0142(19800415)45:8<2198::aid-cncr2820450832>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


