Abstract
The potential of cis-diamminedichloroplatinum(II) (CDDP), trans-di(2-nitroimidazole)dichloro-platinum(II) (NIPt), trans-di(2-amino-5-nitrothiazole)dichloroplatinum(II) (Plant), cis-(1,2-diamino-4-nitrobenzene)dichloroplatinum(II) (Plato), and cis-di-pyridinedichloroplatinum(II) (PyPt) to act as chemosensitizers of mitomycin C cytotoxicity toward EMT6 cells under oxygenated and hypoxic conditions has been assessed. Cells were given a 1 h treatment with the platinum complex under oxygenated or hypoxic conditions and then an additional one hour of exposure to the combination. Two concentrations of each platinum complex, 0.1 and 0.01 microM, were tested in combination with mitomycin C at 1, 0.1 and 0.01 microM. The results were analyzed via isobolograms. Under oxygenated conditions the combinations of the various platinum complexes and mitomycin C produced approximately a 2-3-fold enhancement in cell killing. Under hypoxic conditions enhancements of 5-fold, 20-fold and 60-fold were obtained with CDDP and 1, 0.1 and 0.01 microM mitomycin C, respectively. The combinations of 0.1 microM NIPT and mitomycin C under hypoxic conditions were 30-60-fold more cytotoxic than expected by additivity. With 0.01 microM NIPT a 15-23-fold enhancement of mitomycin C cytotoxicity was observed. The Plant-mitomycin C combinations produced a 5-14-fold enhancement in cell killing under hypoxic conditions. Under hypoxic conditions the combinations of 0.1 microM Plato and mitomycin C were 30-60-fold more cytotoxic than expected. At 0.01 microM Plato an 8-16-fold enhancement in cytotoxicity was observed under hypoxic conditions. PyPt and mitomycin C produced an 8-16-fold enhancement in cytotoxicity under hypoxic conditions. Overall, the platinum complexes containing radiosensitizing nitroaromatic groups were no more active in producing enhanced effects than cis-diamminedichloroplatinum(II).
Full text
PDF

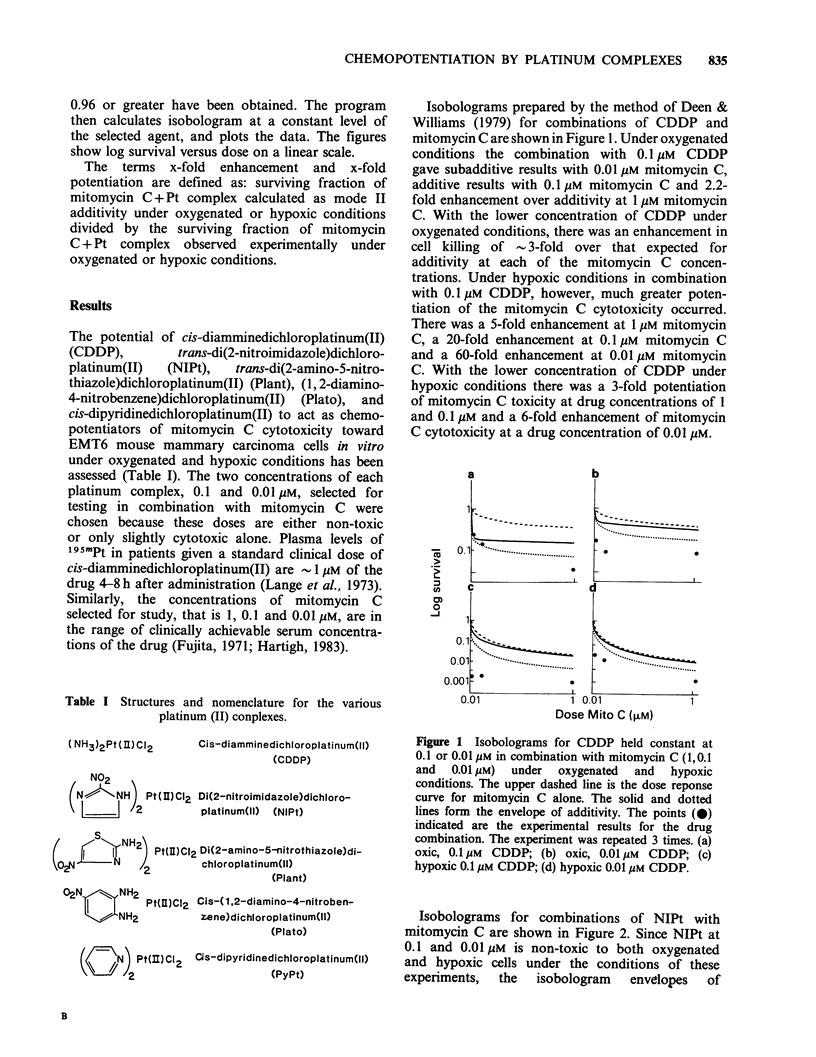
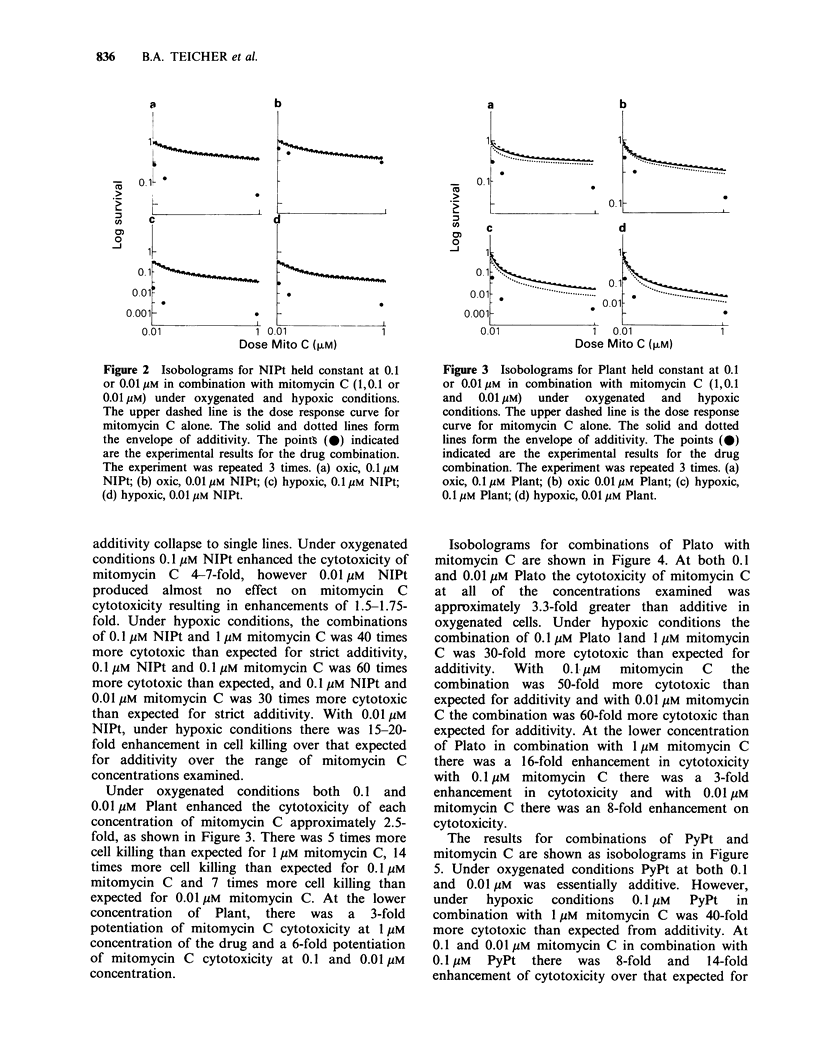
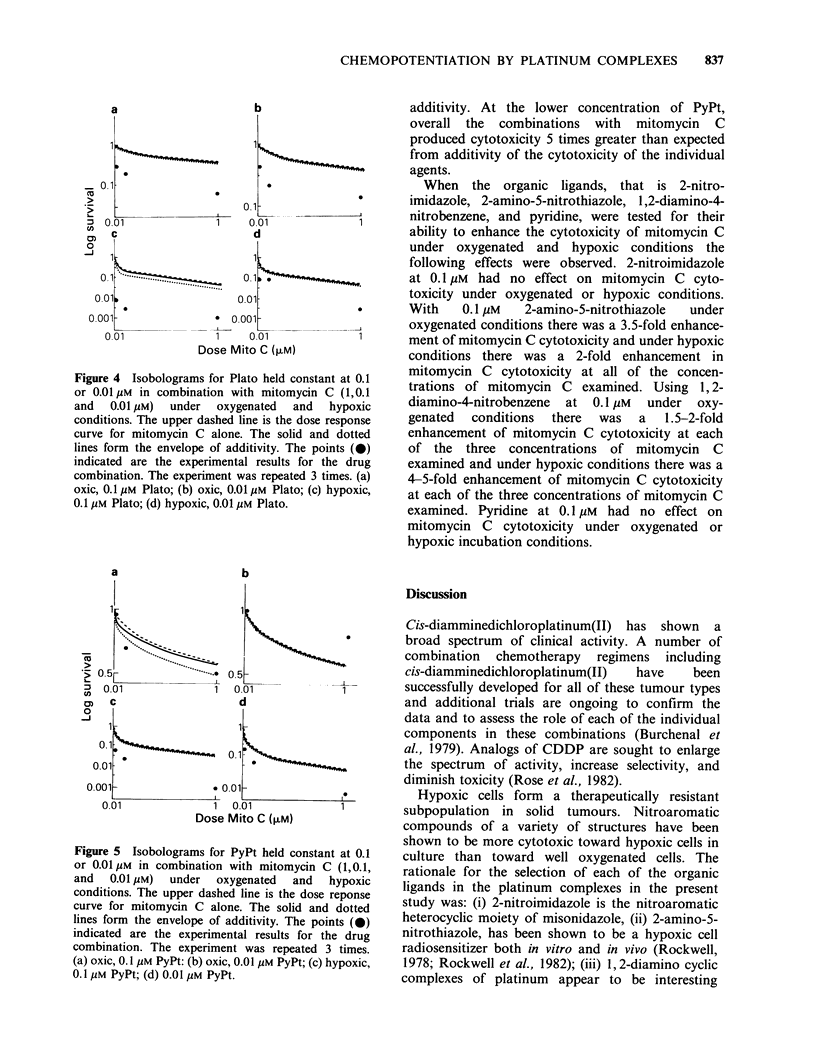


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berenbaum M. C. Synergy, additivism and antagonism in immunosuppression. A critical review. Clin Exp Immunol. 1977 Apr;28(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- Burchenal J. H., Kalaher K., Dew K., Lokys L. Rationale for development of platinum analogs. Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1493–1498. [PubMed] [Google Scholar]
- Clement J. J., Gorman M. S., Wodinsky I., Catane R., Johnson R. K. Enhancement of antitumor activity of alkylating agents by the radiation sensitizer misonidazole. Cancer Res. 1980 Nov;40(11):4165–4172. [PubMed] [Google Scholar]
- Crooke S. T., Bradner W. T. Mitomycin C: a review. Cancer Treat Rev. 1976 Sep;3(3):121–139. doi: 10.1016/s0305-7372(76)80019-9. [DOI] [PubMed] [Google Scholar]
- Deen D. F., Williams M. E. Isobologram analysis of X-ray--BCNU interactions in vitro. Radiat Res. 1979 Sep;79(3):483–491. [PubMed] [Google Scholar]
- Dewey W. C., Stone L. E., Miller H. H., Giblak R. E. Radiosensitization with 5-bromodeoxyuridine of Chinese hamster cells x-irradiated during different phases of the cell cycle. Radiat Res. 1971 Sep;47(3):672–688. [PubMed] [Google Scholar]
- Douple E. B., Richmond R. C. A review of platinum complex biochemistry suggests a rationale for combined platinum-radiotherapy. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1335–1339. doi: 10.1016/0360-3016(79)90665-5. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Loeb E., Pardue A., Khan A., King J. J., Aleman C., Hill N. O. Platinum analogs of clinical interest. Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1509–1513. [PubMed] [Google Scholar]
- Kennedy K. A., Rockwell S., Sartorelli A. C. Preferential activation of mitomycin C to cytotoxic metabolites by hypoxic tumor cells. Cancer Res. 1980 Jul;40(7):2356–2360. [PubMed] [Google Scholar]
- Lange R. C., Spencer R. P., Harder H. C. The antitumor agent cis-Pt(NH 3 ) 2 Cl 2 : distribution studies and dose calculations for 193m Pt and 195m Pt. J Nucl Med. 1973 Apr;14(4):191–195. [PubMed] [Google Scholar]
- Overgaard J., Khan A. R. Selective enhancement of radiation response in a C3H mammary carcinoma by cisplatin. Cancer Treat Rep. 1981 May-Jun;65(5-6):501–503. [PubMed] [Google Scholar]
- Rauth A. M., Mohindra J. K., Tannock I. F. Activity of mitomycin C for aerobic and hypoxic cells in vitro and in vivo. Cancer Res. 1983 Sep;43(9):4154–4158. [PubMed] [Google Scholar]
- Rockwell S. C., Kallman R. F., Fajardo L. F. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J Natl Cancer Inst. 1972 Sep;49(3):735–749. [PubMed] [Google Scholar]
- Rockwell S. Cytotoxic and radiosensitizing effects of hypoxic cell sensitizers on EMT6 mouse mammary tumour cells in vivo and in vitro. Br J Cancer Suppl. 1978 Jun;3:212–215. [PMC free article] [PubMed] [Google Scholar]
- Rockwell S. Cytotoxicities of mitomycin C and x rays to aerobic and hypoxic cells in vitro. Int J Radiat Oncol Biol Phys. 1982 Jun;8(6):1035–1039. doi: 10.1016/0360-3016(82)90173-0. [DOI] [PubMed] [Google Scholar]
- Rockwell S. In vivo-in vitro tumor systems: new models for studing the response of tumours to therapy. Lab Anim Sci. 1977 Oct;27(5 Pt 2):831–851. [PubMed] [Google Scholar]
- Rockwell S., Kallman R. F. Cellular radiosensitivity and tumor radiation response in the EMT6 tumor cell system. Radiat Res. 1973 Feb;53(2):281–294. [PubMed] [Google Scholar]
- Rockwell S., Kennedy K. A. Combination therapy with radiation and mitomycin C: preliminary results with EMT6 tumor cells in vitro and in vivo. Int J Radiat Oncol Biol Phys. 1979 Sep;5(9):1673–1676. doi: 10.1016/0360-3016(79)90795-8. [DOI] [PubMed] [Google Scholar]
- Rockwell S., Mroczkowski Z., Rupp W. D. Evaluation of 2-amino-5-nitrothiazole as a hypoxic cell radiosensitizer. Radiat Res. 1982 Jun;90(3):575–585. [PubMed] [Google Scholar]
- Rose W. C., Schurig J. E., Huftalen J. B., Bradner W. T. Antitumor activity and toxicity of cisplatin analogs. Cancer Treat Rep. 1982 Jan;66(1):135–146. [PubMed] [Google Scholar]
- Schabel F. M., Jr, Trader M. W., Laster W. R., Jr, Corbett T. H., Griswold D. P., Jr cis-Dichlorodiammineplatinum(II): combination chemotherapy and cross-resistance studies with tumors of mice. Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1459–1473. [PubMed] [Google Scholar]
- Steel G. G., Peckham M. J. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys. 1979 Jan;5(1):85–91. doi: 10.1016/0360-3016(79)90044-0. [DOI] [PubMed] [Google Scholar]
- Teicher B. A., Lazo J. S., Sartorelli A. C. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981 Jan;41(1):73–81. [PubMed] [Google Scholar]
- Teicher B. A., Sartorelli A. C. Nitrobenzyl halides and carbamates as prototype bioreductive alkylating agents. J Med Chem. 1980 Aug;23(8):955–960. doi: 10.1021/jm00182a027. [DOI] [PubMed] [Google Scholar]
- Veselý J. Synergistic effect of cis-dichlorodiammineplatinum and 5-aza-2'-deoxycytidine on mouse leukemic cells in vivo and in vitro. Int J Cancer. 1982 Jan 15;29(1):81–85. doi: 10.1002/ijc.2910290114. [DOI] [PubMed] [Google Scholar]


