Abstract
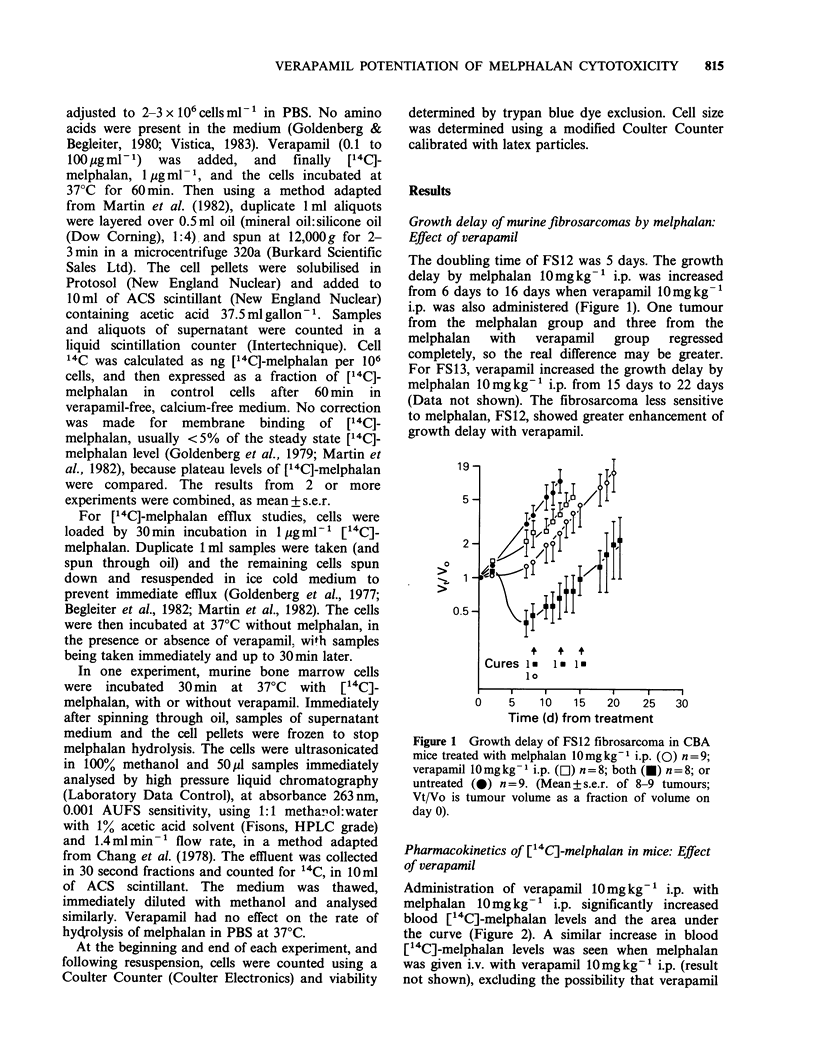
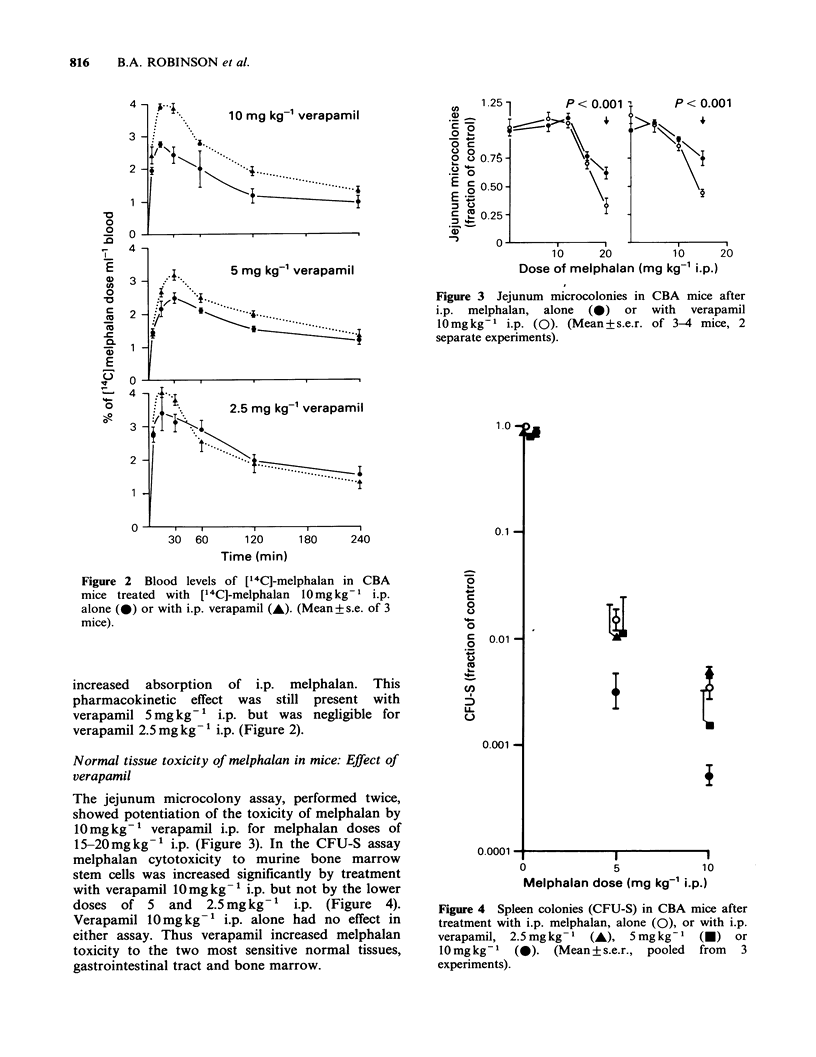
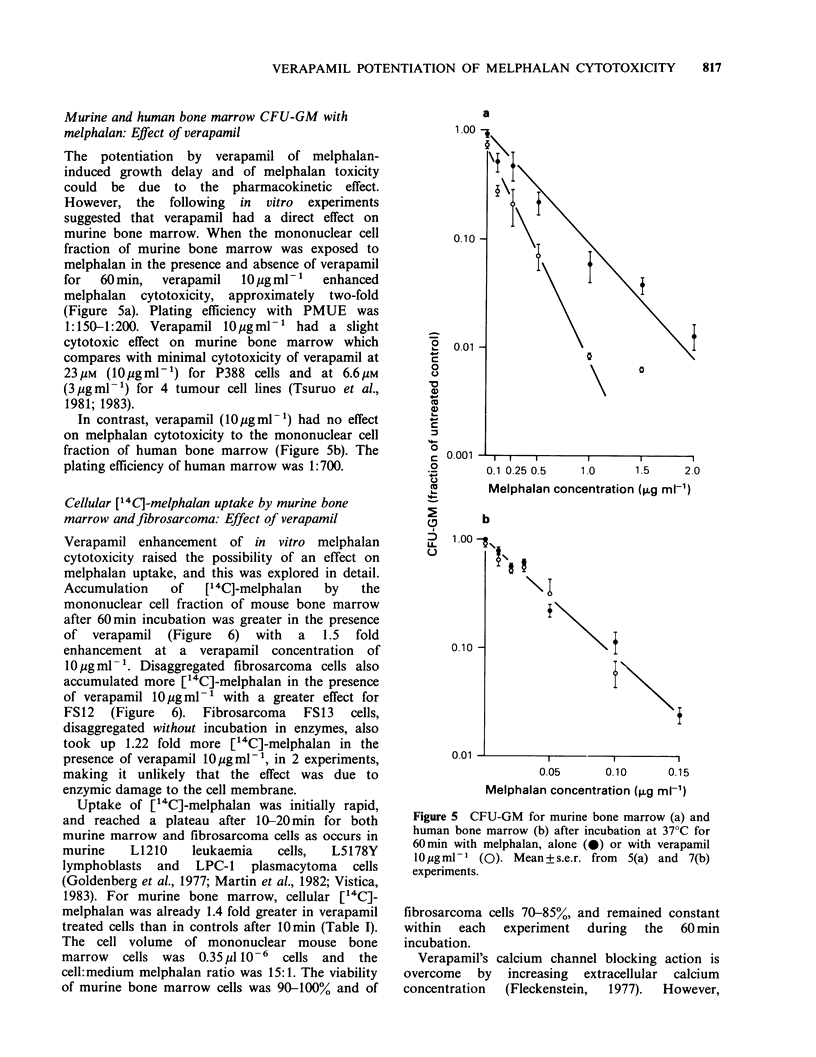
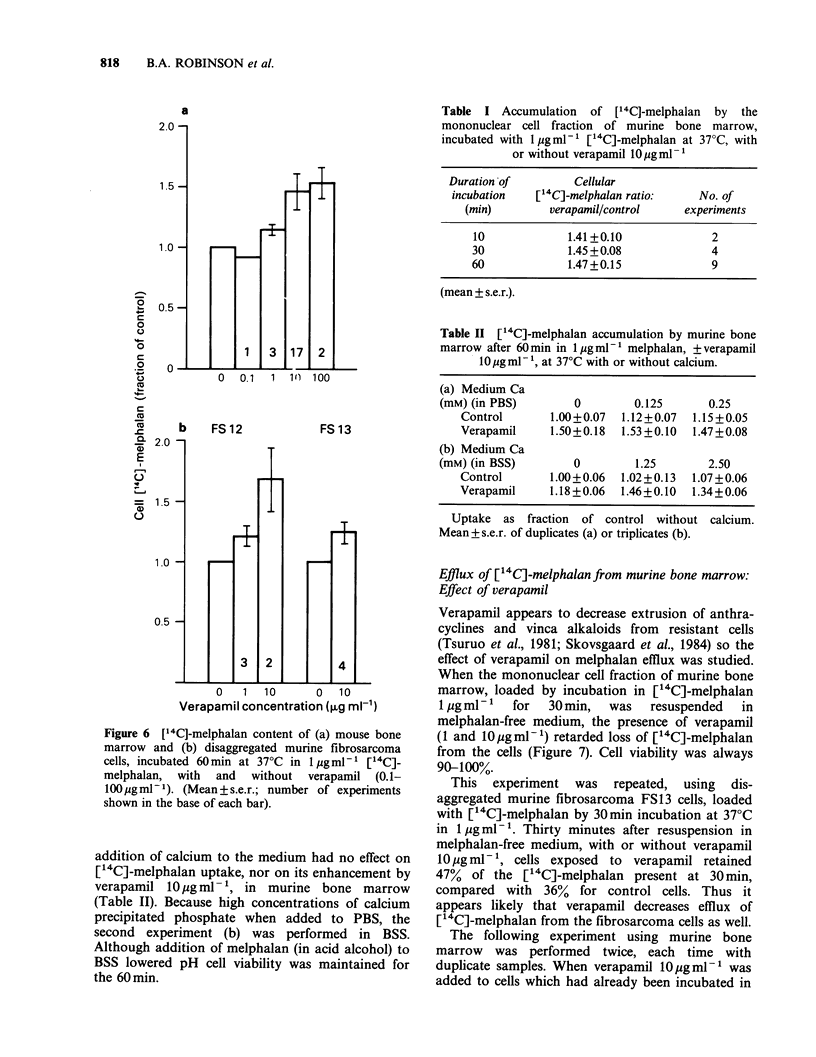
Growth delay by melphalan of two fibrosarcomas in CBA mice was prolonged by intraperitoneal (i.p.) verapamil, 10 mg kg-1. Verapamil also increased the area under the blood concentration time curve and the gastrointestinal toxicity of melphalan. Verapamil promoted melphalan cytotoxicity to murine bone marrow both in vivo, by CFU-S assay, and in vitro, by CFU-GM assay. In 1 microgram ml-1 [14C]-melphalan, verapamil (10 micrograms ml-1) increased by 1.5 times the [14C]-melphalan accumulation by murine bone marrow, reversibly and independently of external calcium. Efflux of [14C]-melphalan from murine bone marrow was retarded by verapamil. Verapamil increased [14C]-melphalan uptake by disaggregated fibrosarcoma cells but had no effect on melphalan accumulation and cytotoxicity in human bone marrow. Although verapamil affected melphalan pharmacokinetics, enhancement of cellular melphalan uptake by verapamil in murine fibrosarcoma and bone marrow appeared to account for much of the increase in melphalan cytotoxicity. The lack of potentiation of melphalan by verapamil in human marrow suggests differences in melphalan transport or in verapamil membrane interactions in mouse and man.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. T. Cellular pharmacology of Vinca alkaloid resistance and its circumvention. Adv Enzyme Regul. 1984;22:207–227. doi: 10.1016/0065-2571(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Froese E. K., Goldenberg G. J. A comparison of melphalan transport in human breast cancer cells and lymphocytes in vitro. Cancer Lett. 1980 Sep;10(3):243–251. doi: 10.1016/0304-3835(80)90077-4. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Grover J., Goldenberg G. J. Mechanism of efflux of melphalan from L5178Y lymphoblasts in vitro. Cancer Res. 1982 Mar;42(3):987–991. [PubMed] [Google Scholar]
- Begleiter A., Lam H. Y., Grover J., Froese E., Goldenberg G. J. Evidence for active transport of melphalan by two amino acid carriers in L5178Y lymphoblasts in vitro. Cancer Res. 1979 Feb;39(2 Pt 1):353–359. [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Chang S. Y., Alberts D. S., Melnick L. R., Walson P. D., Salmon S. E. High-pressure liquid chromatographic analysis of melphalan in plasma. J Pharm Sci. 1978 May;67(5):679–682. doi: 10.1002/jps.2600670529. [DOI] [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Pajak T. F., McIntyre O. R., Kochwa S., Dosik H. Influence of renal failure on myelosuppressive effects of melphalan: Cancer and Leukemia Group B experience. Cancer Treat Rep. 1982 Mar;66(3):475–481. [PubMed] [Google Scholar]
- Curt G. A., Clendeninn N. J., Chabner B. A. Drug resistance in cancer. Cancer Treat Rep. 1984 Jan;68(1):87–99. [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. J., Begleiter A. Membrane transport of alkylating agents. Pharmacol Ther. 1980;8(2):237–274. doi: 10.1016/0163-7258(80)90048-0. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. J., Lam H. Y., Begleiter A. Active carrier-mediated transport of melphalan by two separate amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J Biol Chem. 1979 Feb 25;254(4):1057–1064. [PubMed] [Google Scholar]
- Goldenberg G. J., Lee M., Lam H. Y., Begleiter A. Evidence for carrier-mediated transport of melphalan by L5178Y lymphoblasts in vitro. Cancer Res. 1977 Mar;37(3):755–760. [PubMed] [Google Scholar]
- Hedley D. W., McElwain T. J., Millar J. L., Gordon M. Y. Acceleration of bone-marrow recovery by pre-treatment with cyclophosphamide in patients receiving high-dose melphalan. Lancet. 1978 Nov 4;2(8097):966–968. doi: 10.1016/s0140-6736(78)92529-1. [DOI] [PubMed] [Google Scholar]
- Inaba M., Fujikura R., Sakurai Y. Active efflux common to vincristine and daunorubicin in vincristine-resistant P388 leukemia. Biochem Pharmacol. 1981 Jul 1;30(13):1863–1865. doi: 10.1016/0006-2952(81)90027-7. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Shrivastav S., Shand D. G., Jirtle R. L. Effect of verapamil on malignant tissue blood flow in SMT-2A tumor-bearing rats. Cancer Res. 1982 Oct;42(10):3944–3949. [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Mode of action of calcium antagonists which alter anthracycline resistance. Biochem Pharmacol. 1984 Apr 1;33(7):1157–1160. doi: 10.1016/0006-2952(84)90533-1. [DOI] [PubMed] [Google Scholar]
- Ling V., Kartner N., Sudo T., Siminovitch L., Riordan J. R. Multidrug-resistance phenotype in Chinese hamster ovary cells. Cancer Treat Rep. 1983 Oct;67(10):869–874. [PubMed] [Google Scholar]
- Maraninchi D., Abecasis M., Gastaut J. A., Sebahoun G., Cahn J. Y., Hervé P., Novakovitch G., Carcassonne Y. High-dose melphalan and autologous bone marrow transplant for relapsed acute leukaemia. Cancer Chemother Pharmacol. 1983;10(2):109–111. doi: 10.1007/BF00446220. [DOI] [PubMed] [Google Scholar]
- Martin A. D., Beer R. W., Bosanquet A. G., Gilby E. D. The effect of alkylating agents and other drugs on the accumulation of melphalan by murine L1210 leukaemia cells in vitro. Biochem Pharmacol. 1982 Sep 1;31(17):2727–2732. doi: 10.1016/0006-2952(82)90125-3. [DOI] [PubMed] [Google Scholar]
- McElwain T. J., Powles R. L. High-dose intravenous melphalan for plasma-cell leukaemia and myeloma. Lancet. 1983 Oct 8;2(8354):822–824. doi: 10.1016/s0140-6736(83)90739-0. [DOI] [PubMed] [Google Scholar]
- McMahon M. T., Sheaffer S. L. Verapamil (Isoptin, Knoll; Calan, Searle). Drug Intell Clin Pharm. 1982 Jun;16(6):443–447. doi: 10.1177/106002808201600601. [DOI] [PubMed] [Google Scholar]
- Millar J. L., Blackett N. M., Hudspith B. N. Enhanced post-irradiation recovery of the haemopoietic system in animals pretreated with a variety of cytotoxic agents. Cell Tissue Kinet. 1978 Sep;11(5):543–553. doi: 10.1111/j.1365-2184.1978.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Millar J. L., Hudspith B. N., McElwain T. J., Phelps T. A. Effect of high-dose melphalan on marrow and intestinal epithelium in mice pretreated with cyclophosphamide. Br J Cancer. 1978 Jul;38(1):137–142. doi: 10.1038/bjc.1978.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. L., Phelps T. A., Carter R. L., McElwain T. J. Cyclophosphamide pretreatment reduces the toxic effect of high dose melphalan on intestinal epithelium in sheep. Eur J Cancer. 1978 Nov;14(11):1283–1285. doi: 10.1016/0014-2964(78)90236-0. [DOI] [PubMed] [Google Scholar]
- Murray S. L., Du Vall E. M., Slater L. M. Calcium modifies the accumulation and retention of daunorubicin by Ehrlich ascites carcinoma. Cancer Chemother Pharmacol. 1984;13(1):69–70. doi: 10.1007/BF00401452. [DOI] [PubMed] [Google Scholar]
- Myers C. D., Katz F. E., Joshi G., Millar J. L. A cell line secreting stimulating factors for CFU-GEMM culture. Blood. 1984 Jul;64(1):152–155. [PubMed] [Google Scholar]
- Pritchard J., McElwain T. J., Graham-Pole J. High-dose melphalan with autologous marrow for treatment of advanced neuroblastoma. Br J Cancer. 1982 Jan;45(1):86–94. doi: 10.1038/bjc.1982.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Weintraub H. Differences in lipid composition of doxorubicin-sensitive and -resistant P388 cells. Cancer Treat Rep. 1984 Apr;68(4):637–641. [PubMed] [Google Scholar]
- Redwood W. R., Colvin M. Transport of melphalan by sensitive and resistant L1210 cells. Cancer Res. 1980 Apr;40(4):1144–1149. [PubMed] [Google Scholar]
- Rogan A. M., Hamilton T. C., Young R. C., Klecker R. W., Jr, Ozols R. F. Reversal of adriamycin resistance by verapamil in human ovarian cancer. Science. 1984 Jun 1;224(4652):994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- Simpson W. G., Tseng M. T., Anderson K. C., Harty J. I. Verapamil enhancement of chemotherapeutic efficacy in human bladder cancer cells. J Urol. 1984 Sep;132(3):574–576. doi: 10.1016/s0022-5347(17)49749-7. [DOI] [PubMed] [Google Scholar]
- Slater L. M., Murray S. L., Wetzel M. W., Wisdom R. M., DuVall E. M. Verapamil restoration of daunorubicin responsiveness in daunorubicin-resistant Ehrlich ascites carcinoma. J Clin Invest. 1982 Nov;70(5):1131–1134. doi: 10.1172/JCI110702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Naganuma K., Tsukagoshi S., Sakurai Y. Promotion by verapamil of vincristine responsiveness in tumor cell lines inherently resistant to the drug. Cancer Res. 1983 Feb;43(2):808–813. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]
- Vistica D. T. Cellular pharmacokinetics of the phenylalanine mustards. Pharmacol Ther. 1983;22(3):379–406. doi: 10.1016/0163-7258(83)90009-8. [DOI] [PubMed] [Google Scholar]
- Vistica D. T. Cytotoxicity as an indicator for transport mechanism: evidence that murine bone marrow progenitor cells lack a high-affinity leucine carrier that transports melphalan in murine L1210 leukemia cells. Blood. 1980 Sep;56(3):427–429. [PubMed] [Google Scholar]
- Withers H. R., Elkind M. M. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- Yalowich J. C., Ross W. E. Potentiation of etoposide-induced DNA damage by calcium antagonists in L1210 cells in vitro. Cancer Res. 1984 Aug;44(8):3360–3365. [PubMed] [Google Scholar]
- Yalowich J. C., Ross W. E. Verapamil-induced augmentation of etoposide accumulation in L1210 cells in vitro. Cancer Res. 1985 Apr;45(4):1651–1656. [PubMed] [Google Scholar]
- Yanovich S., Preston L. Effects of verapamil on daunomycin cellular retention and cytotoxicity in P388 leukemic cells. Cancer Res. 1984 May;44(5):1743–1747. [PubMed] [Google Scholar]


