Abstract
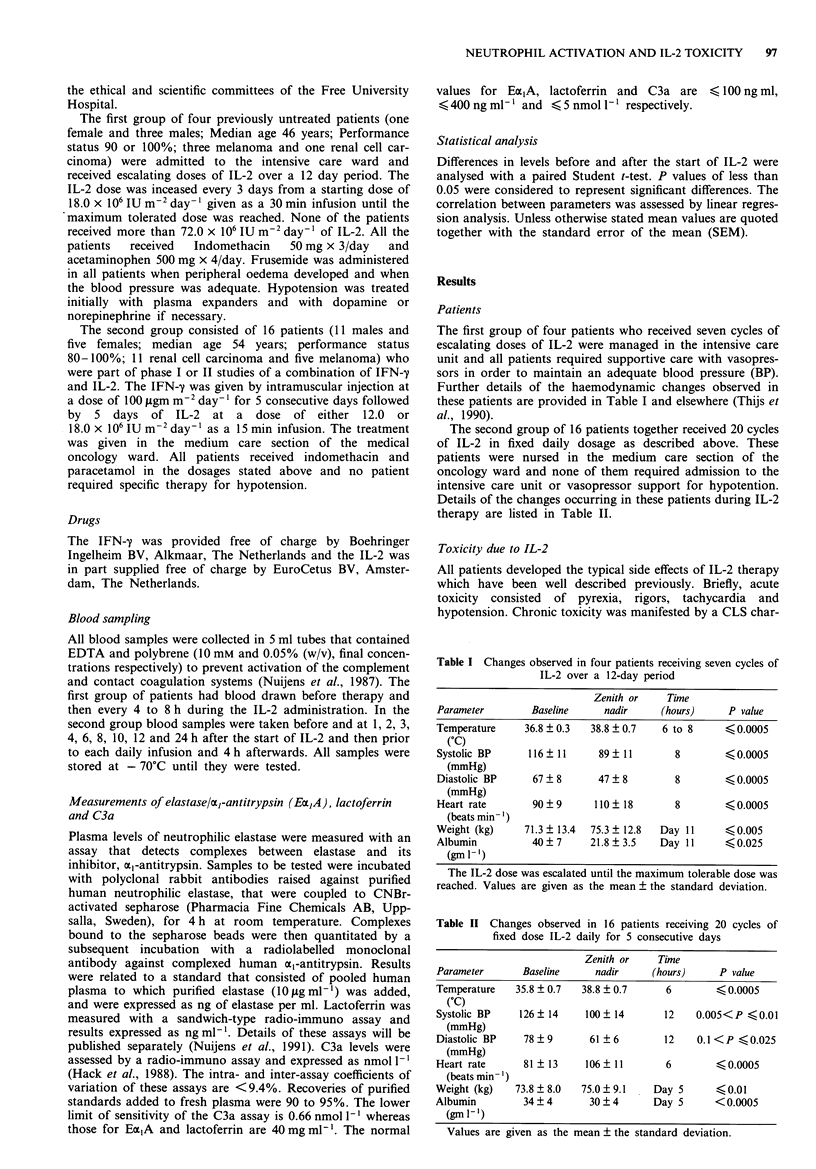
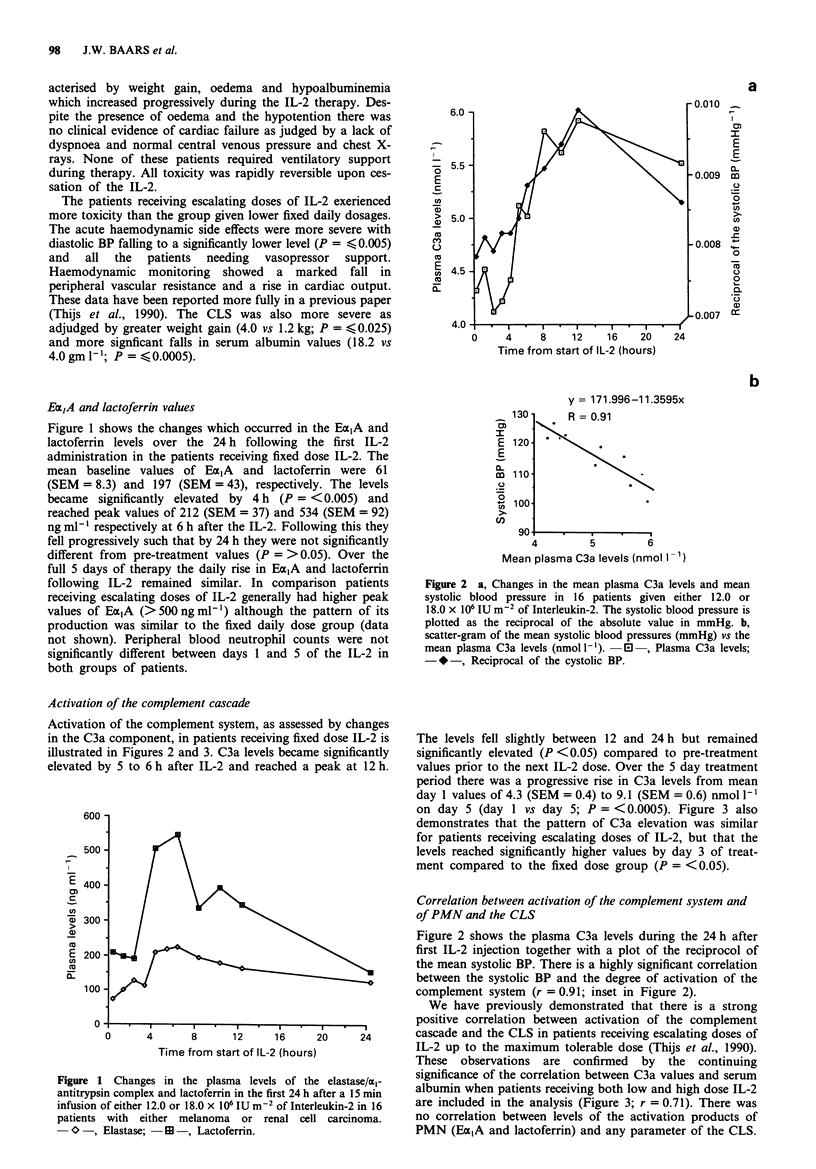
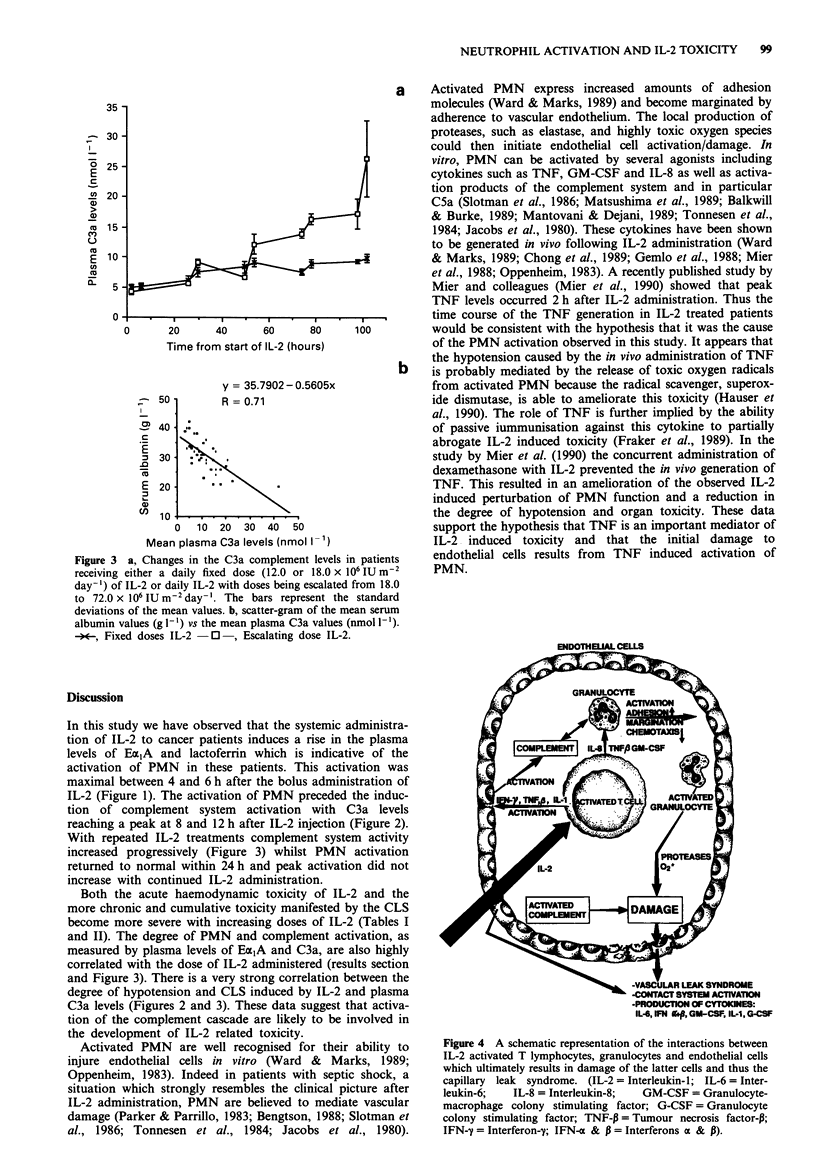
The toxicity due to interleukin-2 (IL-2) strongly resembles the clinical picture seen during septic shock. In septic shock activation of polymorphonuclear neutrophils (PMN) and the complement system contribute significantly to the pathophysiology of the condition. We therefore investigated whether similar events contributed to the toxicity observed with IL-2. Four patients received seven cycles of escalating dose IL-2 (18.0 to 72.0 X 10(6) IU m-2 day-1) and 16 were treated with 20 cycles of fixed dose IL-2 (12.0 or 18.0 X 10(6) IU m-2 day-1). Toxicity, as judged by hypotension (P = less than 0.005) and capillary leakage (fall in serum albumin 18.2 vs 4.0 gm l-1; P = less than 0.0005 and weight gain 4.0 vs 1.2 kg; P = less than 0.025) were worse with the esc. dose protocol. PMN became activated following IL-2 with mean peak elastase/alpha 1-antitrypsin (E alpha 1 A) and lactoferrin values of 212 (SEM = 37) and 534 (SEM = 92) ng ml-1 respectively occurring 6 h after the IL-2. Peak values for the esc. dose IL-2 group being generally higher than 500 ng ml-1. Activation of the complement cascade was evidenced by a dose dependent elevation of peak C3a values (fixed dose 9.1 (SEM = 0.6); esc. dose 25.7 (SEM = 6.33); P = less than 0.005) on day 5 of IL-2. There was a significant correlation between C3a levels and the degree of hypotention during the first 24 h after IL-2 (r = 0.91) and parameters of capillary leakage such as weight gain and fall in serum albumin (r = 0.71). These data suggest that activation of PMN initiates endothelial cell damage which subsequently leads to activation of the complement cascade. This latter system then contributes to the haemodynamic changes and capillary leakage seen in IL-2 treated patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson F. R., Libby P., Brandon E. P., Janicka M. W., Mier J. W. IL-2 rapidly induces natural killer cell adhesion to human endothelial cells. A potential mechanism for endothelial injury. J Immunol. 1988 Jul 1;141(1):158–163. [PubMed] [Google Scholar]
- Balkwill F. R., Burke F. The cytokine network. Immunol Today. 1989 Sep;10(9):299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- Bengtson A., Heideman M. Anaphylatoxin formation in sepsis. Arch Surg. 1988 May;123(5):645–649. doi: 10.1001/archsurg.1988.01400290131023. [DOI] [PubMed] [Google Scholar]
- Chong A. S., Scuderi P., Grimes W. J., Hersh E. M. Tumor targets stimulate IL-2 activated killer cells to produce interferon-gamma and tumor necrosis factor. J Immunol. 1989 Mar 15;142(6):2133–2139. [PubMed] [Google Scholar]
- Cotran R. S., Pober J. S. Effects of cytokines on vascular endothelium: their role in vascular and immune injury. Kidney Int. 1989 Apr;35(4):969–975. doi: 10.1038/ki.1989.80. [DOI] [PubMed] [Google Scholar]
- Cotran R. S., Pober J. S., Gimbrone M. A., Jr, Springer T. A., Wiebke E. A., Gaspari A. A., Rosenberg S. A., Lotze M. T. Endothelial activation during interleukin 2 immunotherapy. A possible mechanism for the vascular leak syndrome. J Immunol. 1988 Mar 15;140(6):1883–1888. [PubMed] [Google Scholar]
- Damle N. K., Doyle L. V. IL-2-activated human killer lymphocytes but not their secreted products mediate increase in albumin flux across cultured endothelial monolayers. Implications for vascular leak syndrome. J Immunol. 1989 Apr 15;142(8):2660–2669. [PubMed] [Google Scholar]
- Dutcher J. P., Creekmore S., Weiss G. R., Margolin K., Markowitz A. B., Roper M., Parkinson D., Ciobanu N., Fisher R. I., Boldt D. H. A phase II study of interleukin-2 and lymphokine-activated killer cells in patients with metastatic malignant melanoma. J Clin Oncol. 1989 Apr;7(4):477–485. doi: 10.1200/JCO.1989.7.4.477. [DOI] [PubMed] [Google Scholar]
- Eberlein T. J., Schoof D. D., Jung S. E., Davidson D., Gramolini B., McGrath K., Massaro A., Wilson R. E. A new regimen of interleukin 2 and lymphokine-activated killer cells. Efficacy without significant toxicity. Arch Intern Med. 1988 Dec;148(12):2571–2576. [PubMed] [Google Scholar]
- Fisher R. I., Coltman C. A., Jr, Doroshow J. H., Rayner A. A., Hawkins M. J., Mier J. W., Wiernik P., McMannis J. D., Weiss G. R., Margolin K. A. Metastatic renal cancer treated with interleukin-2 and lymphokine-activated killer cells. A phase II clinical trial. Ann Intern Med. 1988 Apr;108(4):518–523. doi: 10.7326/0003-4819-108-4-518. [DOI] [PubMed] [Google Scholar]
- Fraker D. L., Langstein H. N., Norton J. A. Passive immunization against tumor necrosis factor partially abrogates interleukin 2 toxicity. J Exp Med. 1989 Sep 1;170(3):1015–1020. doi: 10.1084/jem.170.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor E. R., Vitek L., Sticklin L., Creekmore S. P., Ferraro M. E., Thomas J. X., Jr, Fisher S. G., Fisher R. I. The hemodynamic effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med. 1988 Dec 15;109(12):953–958. doi: 10.7326/0003-4819-109-12-953. [DOI] [PubMed] [Google Scholar]
- Gemlo B. T., Palladino M. A., Jr, Jaffe H. S., Espevik T. P., Rayner A. A. Circulating cytokines in patients with metastatic cancer treated with recombinant interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1988 Oct 15;48(20):5864–5867. [PubMed] [Google Scholar]
- Hack C. E., Wagstaff J., Strack van Schijndel R. J., Eerenberg A. J., Pinedo H. M., Thijs L. G., Nuijens J. H. Studies on the contact system of coagulation during therapy with high doses of recombinant IL-2: implications for septic shock. Thromb Haemost. 1991 May 6;65(5):497–503. [PubMed] [Google Scholar]
- Harris R. L., Musher D. M., Bloom K., Gathe J., Rice L., Sugarman B., Williams T. W., Jr, Young E. J. Manifestations of sepsis. Arch Intern Med. 1987 Nov;147(11):1895–1906. [PubMed] [Google Scholar]
- Hauser G. J., McIntosh J. K., Travis W. D., Rosenberg S. A. Manipulation of oxygen radical-scavenging capacity in mice alters host sensitivity to tumor necrosis factor toxicity but does not interfere with its antitumor efficacy. Cancer Res. 1990 Jun 15;50(12):3503–3508. [PubMed] [Google Scholar]
- Jacob H. S., Craddock P. R., Hammerschmidt D. E., Moldow C. F. Complement-induced granulocyte aggregation: an unsuspected mechanism of disease. N Engl J Med. 1980 Apr 3;302(14):789–794. doi: 10.1056/NEJM198004033021407. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Vachino G., Klempner M. S., Aronson F. R., Noring R., Smith S., Brandon E. P., Laird W., Atkins M. B. Inhibition of interleukin-2-induced tumor necrosis factor release by dexamethasone: prevention of an acquired neutrophil chemotaxis defect and differential suppression of interleukin-2-associated side effects. Blood. 1990 Nov 15;76(10):1933–1940. [PubMed] [Google Scholar]
- Mier J. W., Vachino G., van der Meer J. W., Numerof R. P., Adams S., Cannon J. G., Bernheim H. A., Atkins M. B., Parkinson D. R., Dinarello C. A. Induction of circulating tumor necrosis factor (TNF alpha) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J Clin Immunol. 1988 Nov;8(6):426–436. doi: 10.1007/BF00916947. [DOI] [PubMed] [Google Scholar]
- Nuijens J. H., Huijbregts C. C., Cohen M., Navis G. O., de Vries A., Eerenberg A. J., Bakker J. C., Hack C. E. Detection of activation of the contact system of coagulation in vitro and in vivo: quantitation of activated Hageman factor-C-1-inhibitor and kallikrein-C-1-inhibitor complexes by specific radioimmunoassays. Thromb Haemost. 1987 Aug 4;58(2):778–785. [PubMed] [Google Scholar]
- Nuijens J. H., Huijbregts C. C., Eerenberg-Belmer A. J., Abbink J. J., Strack van Schijndel R. J., Felt-Bersma R. J., Thijs L. G., Hack C. E. Quantification of plasma factor XIIa-Cl(-)-inhibitor and kallikrein-Cl(-)-inhibitor complexes in sepsis. Blood. 1988 Dec;72(6):1841–1848. [PubMed] [Google Scholar]
- Ognibene F. P., Rosenberg S. A., Lotze M., Skibber J., Parker M. M., Shelhamer J. H., Parrillo J. E. Interleukin-2 administration causes reversible hemodynamic changes and left ventricular dysfunction similar to those seen in septic shock. Chest. 1988 Oct;94(4):750–754. doi: 10.1378/chest.94.4.750. [DOI] [PubMed] [Google Scholar]
- Oliver R. T. The clinical potential of interleukin-2. Br J Cancer. 1988 Oct;58(4):405–409. doi: 10.1038/bjc.1988.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. M., Parrillo J. E. Septic shock. Hemodynamics and pathogenesis. JAMA. 1983 Dec 23;250(24):3324–3327. [PubMed] [Google Scholar]
- Perez H. D., Ohtani O., Banda D., Ong R., Fukuyama K., Goldstein I. M. Generation of biologically active, complement-(C5) derived peptides by cathepsin H. J Immunol. 1983 Jul;131(1):397–402. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Aebersold P. M., Linehan W. M., Seipp C. A., White D. E. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow W. K., Thong Y. H., McCormack J. G., Ferrante A. Lymphocyte-neutrophil interactions: opposite effects of interleukin-2 and tumour necrosis factor-beta (lymphotoxin) on human neutrophil adherence. Int Arch Allergy Appl Immunol. 1988;85(1):63–68. doi: 10.1159/000234475. [DOI] [PubMed] [Google Scholar]
- Slotman G. J., Burchard K. W., Williams J. J., D'Arezzo A., Yellin S. A. Interaction of prostaglandins, activated complement, and granulocytes in clinical sepsis and hypotension. Surgery. 1986 Jun;99(6):744–751. [PubMed] [Google Scholar]
- Stevens J. H., O'Hanley P., Shapiro J. M., Mihm F. G., Satoh P. S., Collins J. A., Raffin T. A. Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic primates. J Clin Invest. 1986 Jun;77(6):1812–1816. doi: 10.1172/JCI112506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs L. G., Hack C. E., Strack van Schijndel R. J., Nuijens J. H., Wolbink G. J., Eerenberg-Belmer A. J., Van der Vall H., Wagstaff J. Activation of the complement system during immunotherapy with recombinant IL-2. Relation to the development of side effects. J Immunol. 1990 Mar 15;144(6):2419–2424. [PubMed] [Google Scholar]
- Tonnesen M. G., Smedly L. A., Henson P. M. Neutrophil-endothelial cell interactions. Modulation of neutrophil adhesiveness induced by complement fragments C5a and C5a des arg and formyl-methionyl-leucyl-phenylalanine in vitro. J Clin Invest. 1984 Nov;74(5):1581–1592. doi: 10.1172/JCI111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Marks R. M. The acute inflammatory reaction. Curr Opin Immunol. 1989 Oct;2(1):5–9. doi: 10.1016/0952-7915(89)90090-3. [DOI] [PubMed] [Google Scholar]
- di Giovine F. S., Duff G. W. Interleukin 1: the first interleukin. Immunol Today. 1990 Jan;11(1):13–20. doi: 10.1016/0167-5699(90)90005-t. [DOI] [PubMed] [Google Scholar]


