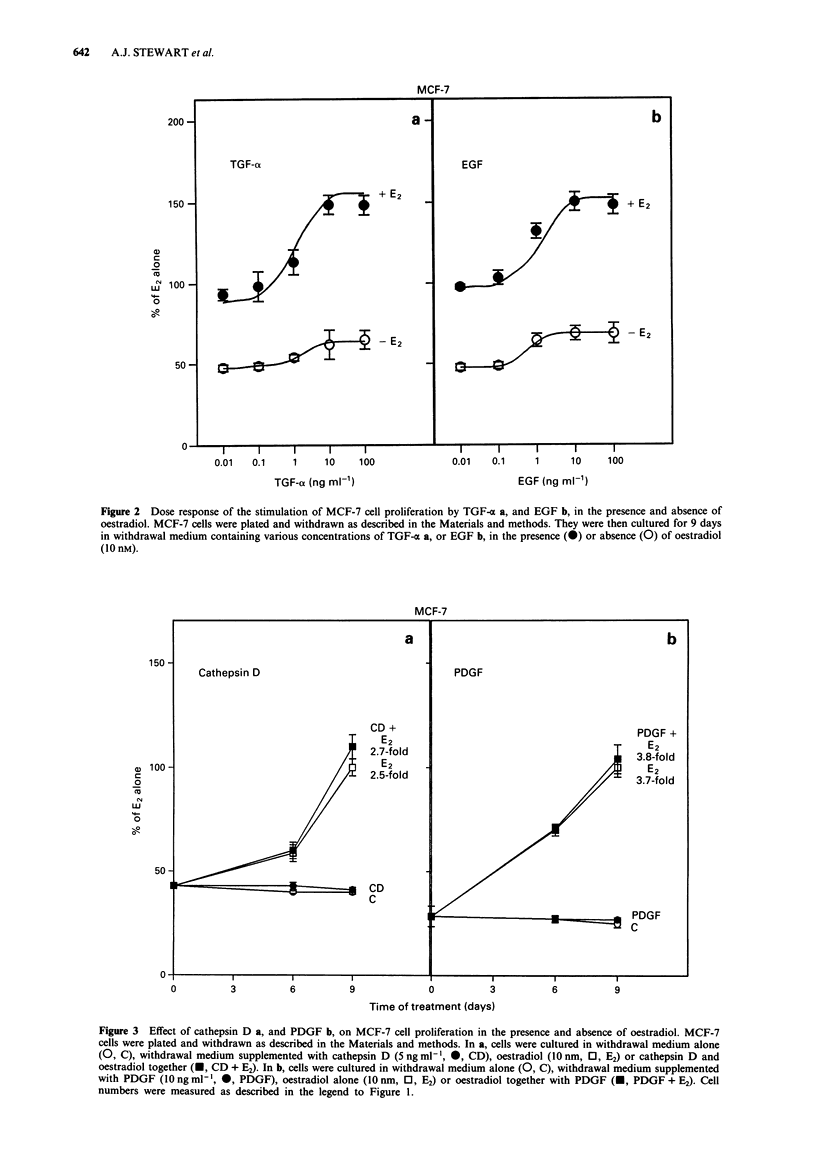
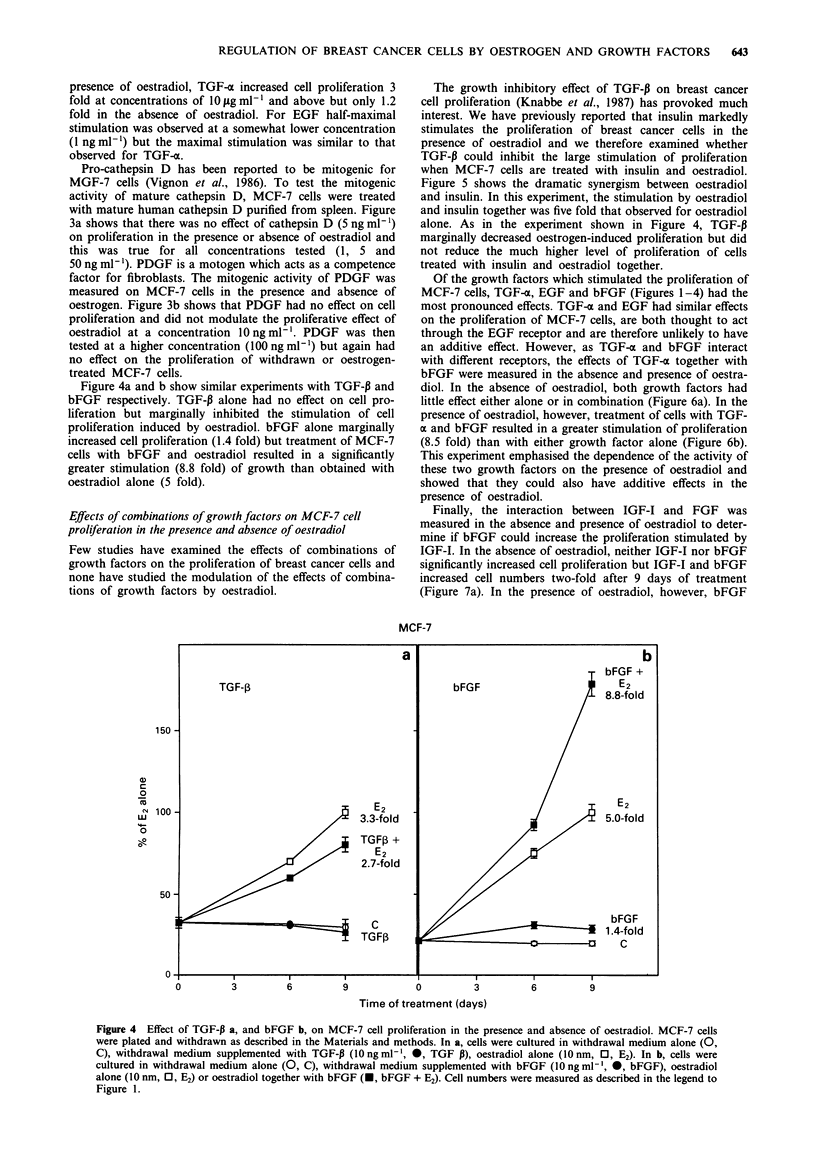
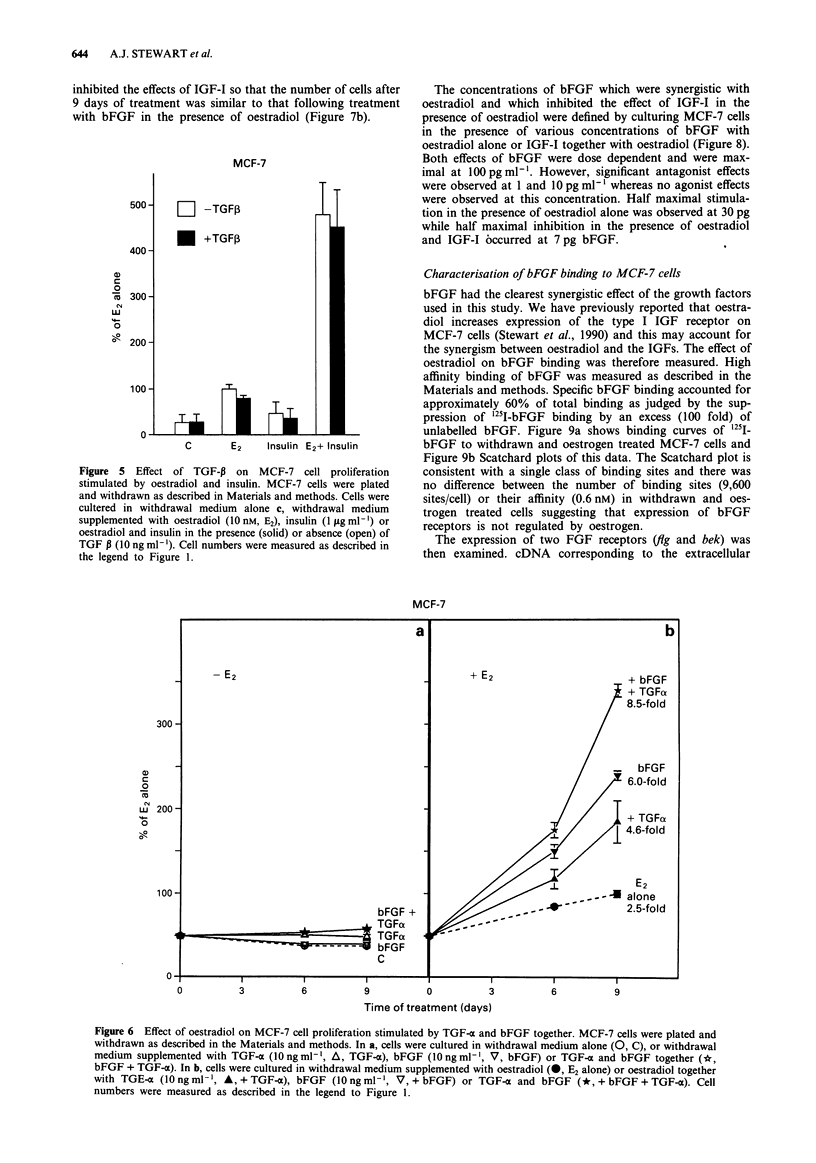
Abstract
A number of growth factors have been implicated in the control of the proliferation of breast cancer cells and some have been reported to mediate the proliferative effects of oestradiol. MCF-7 cells were treated with growth factors in the presence and absence of oestradiol. Oestradiol increased the response of cells to the proliferative effects of epidermal growth factor (EGF), transforming growth factor alpha (TGF-alpha) and basic fibroblast growth factor (bFGF). Platelet derived growth factor (PDGF) and cathepsin D had no effect in the presence or absence of oestradiol while TGF-beta slightly reduced the stimulation by oestradiol. In the absence of oestradiol, there was little effect of combinations of growth factors although the effects of bFGF and IGF-I were additive. In the presence of oestradiol, the effects of bFGF and TGF-alpha were additive whereas bFGF acted as an IGF-I antagonist. Overall, bFGF had the greatest effect on cell proliferation although this was less marked than the previously described effect of the IGFs and insulin. The effects of oestradiol on the sensitivity of cells to the proliferative effects of bFGF did not appear to result from regulation of bFGF receptor expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arteaga C. L., Tandon A. K., Von Hoff D. D., Osborne C. K. Transforming growth factor beta: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res. 1988 Jul 15;48(14):3898–3904. [PubMed] [Google Scholar]
- Bates S. E., Davidson N. E., Valverius E. M., Freter C. E., Dickson R. B., Tam J. P., Kudlow J. E., Lippman M. E., Salomon D. S. Expression of transforming growth factor alpha and its messenger ribonucleic acid in human breast cancer: its regulation by estrogen and its possible functional significance. Mol Endocrinol. 1988 Jun;2(6):543–555. doi: 10.1210/mend-2-6-543. [DOI] [PubMed] [Google Scholar]
- Bronzert D. A., Pantazis P., Antoniades H. N., Kasid A., Davidson N., Dickson R. B., Lippman M. E. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5763–5767. doi: 10.1073/pnas.84.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R., Brünner N., Katz D., Glanz P., Dickson R. B., Lippman M. E., Kern F. G. The effects of a constitutive expression of transforming growth factor-alpha on the growth of MCF-7 human breast cancer cells in vitro and in vivo. Mol Endocrinol. 1989 Feb;3(2):372–380. doi: 10.1210/mend-3-2-372. [DOI] [PubMed] [Google Scholar]
- Cullen K. J., Yee D., Bates S. E., Brunner N., Clarke R., Dickson R. E., Huff K. K., Paik S., Rosen N., Valverius E. Regulation of human breast cancer by secreted growth factors. Acta Oncol. 1989;28(6):835–839. doi: 10.3109/02841868909092318. [DOI] [PubMed] [Google Scholar]
- Dionne C. A., Crumley G., Bellot F., Kaplow J. M., Searfoss G., Ruta M., Burgess W. H., Jaye M., Schlessinger J. Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors. EMBO J. 1990 Sep;9(9):2685–2692. doi: 10.1002/j.1460-2075.1990.tb07454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. D., Westley B. R., May F. E. Oestrogenic activity of tamoxifen and its metabolites on gene regulation and cell proliferation in MCF-7 breast cancer cells. Br J Cancer. 1989 May;59(5):727–738. doi: 10.1038/bjc.1989.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan M., DiSorbo D., Hou J. Z., Hoshi H., Mansson P. E., McKeehan W. L. High and low affinity binding of heparin-binding growth factor to a 130-kDa receptor correlates with stimulation and inhibition of growth of a differentiated human hepatoma cell. J Biol Chem. 1988 Aug 15;263(23):11306–11313. [PubMed] [Google Scholar]
- Karey K. P., Sirbasku D. A. Differential responsiveness of human breast cancer cell lines MCF-7 and T47D to growth factors and 17 beta-estradiol. Cancer Res. 1988 Jul 15;48(14):4083–4092. [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Leung B. S., Potter A. H. Mode of estrogen action on cell proliferation in CAMA-1 cells: II. Sensitivity of G1 phase population. J Cell Biochem. 1987 Jul;34(3):213–225. doi: 10.1002/jcb.240340307. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B., Gelmann E. P., Rosen N., Knabbe C., Bates S., Bronzert D., Huff K., Kasid A. Growth regulatory peptide production by human breast carcinoma cells. J Steroid Biochem. 1988;30(1-6):53–61. doi: 10.1016/0022-4731(88)90076-3. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- May F. E., Westley B. R. Identification and characterization of estrogen-regulated RNAs in human breast cancer cells. J Biol Chem. 1988 Sep 15;263(26):12901–12908. [PubMed] [Google Scholar]
- Minuto F., Del Monte P., Barreca A., Alama A., Cariola G., Giordano G. Evidence for autocrine mitogenic stimulation by somatomedin-C/insulin-like growth factor I on an established human lung cancer cell line. Cancer Res. 1988 Jul 1;48(13):3716–3719. [PubMed] [Google Scholar]
- Osborne C. K., Coronado E. B., Kitten L. J., Arteaga C. I., Fuqua S. A., Ramasharma K., Marshall M., Li C. H. Insulin-like growth factor-II (IGF-II): a potential autocrine/paracrine growth factor for human breast cancer acting via the IGF-I receptor. Mol Endocrinol. 1989 Nov;3(11):1701–1709. doi: 10.1210/mend-3-11-1701. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Yochmowitz M. G., Knight W. A., 3rd, McGuire W. L. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980 Dec 15;46(12 Suppl):2884–2888. doi: 10.1002/1097-0142(19801215)46:12+<2884::aid-cncr2820461429>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Reid W. A., Valler M. J., Kay J. Immunolocalization of cathepsin D in normal and neoplastic human tissues. J Clin Pathol. 1986 Dec;39(12):1323–1330. doi: 10.1136/jcp.39.12.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudland P. S., Hallowes R. C., Durbin H., Lewis D. Mitogenic activity of pituitary hormones on cell cultures of normal and carcinogen-induced tumor epithelium from rat mammary glands. J Cell Biol. 1977 Jun;73(3):561–577. doi: 10.1083/jcb.73.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Winslow D. P., Rudland P. S. Different growth factors stimulate cell division of rat mammary epithelial, myoepithelial, and stromal cell lines in culture. J Cell Physiol. 1984 Jun;119(3):320–326. doi: 10.1002/jcp.1041190310. [DOI] [PubMed] [Google Scholar]
- Stewart A. J., Johnson M. D., May F. E., Westley B. R. Role of insulin-like growth factors and the type I insulin-like growth factor receptor in the estrogen-stimulated proliferation of human breast cancer cells. J Biol Chem. 1990 Dec 5;265(34):21172–21178. [PubMed] [Google Scholar]
- Vignon F., Capony F., Chambon M., Freiss G., Garcia M., Rochefort H. Autocrine growth stimulation of the MCF 7 breast cancer cells by the estrogen-regulated 52 K protein. Endocrinology. 1986 Apr;118(4):1537–1545. doi: 10.1210/endo-118-4-1537. [DOI] [PubMed] [Google Scholar]
- Yee D., Rosen N., Favoni R. E., Cullen K. J. The insulin-like growth factors, their receptors, and their binding proteins in human breast cancer. Cancer Treat Res. 1991;53:93–106. doi: 10.1007/978-1-4615-3940-7_5. [DOI] [PubMed] [Google Scholar]