Abstract
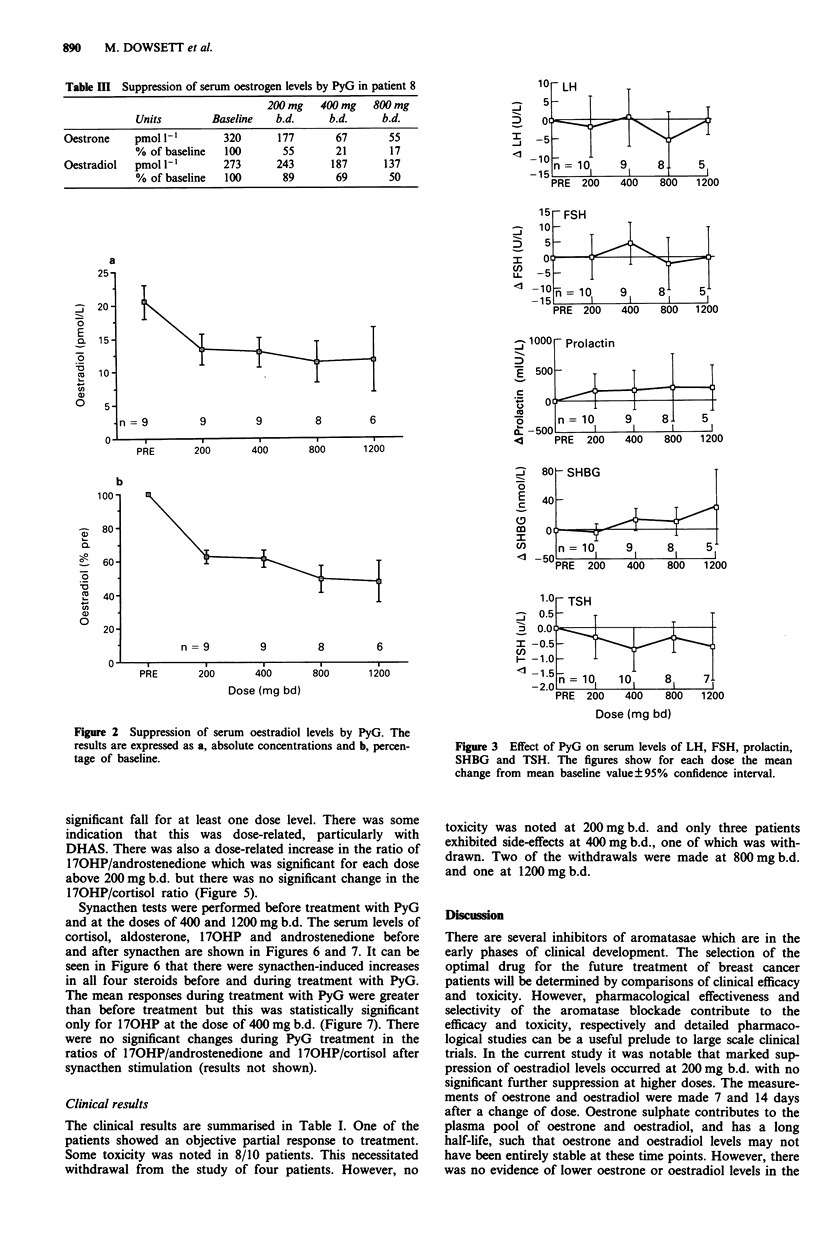
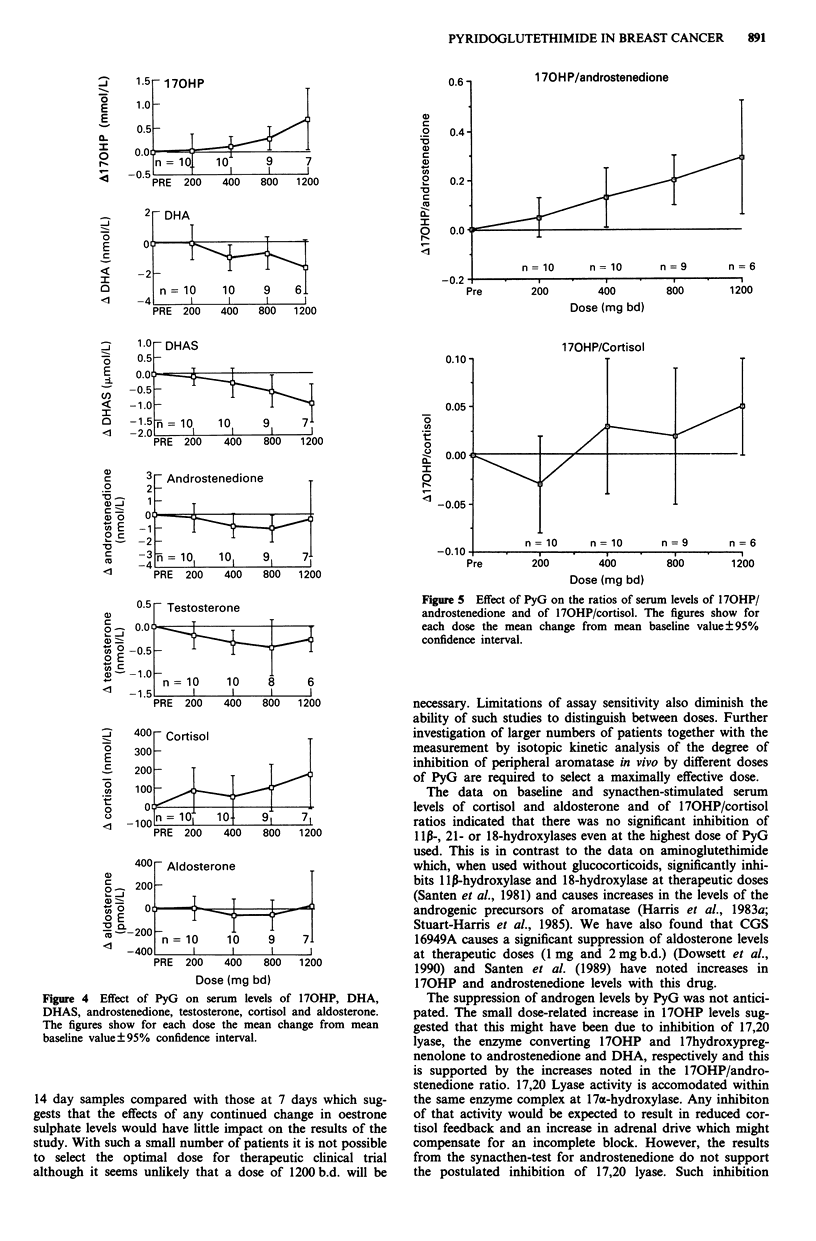
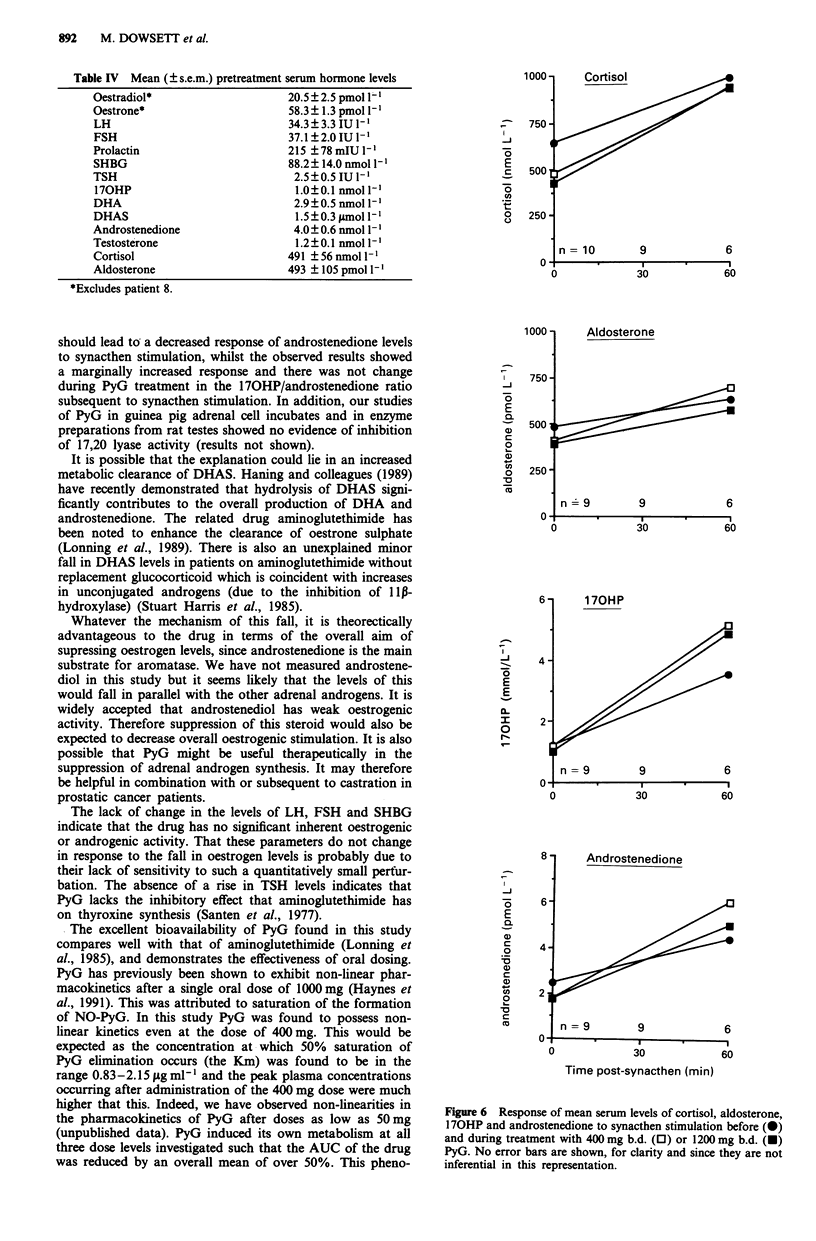
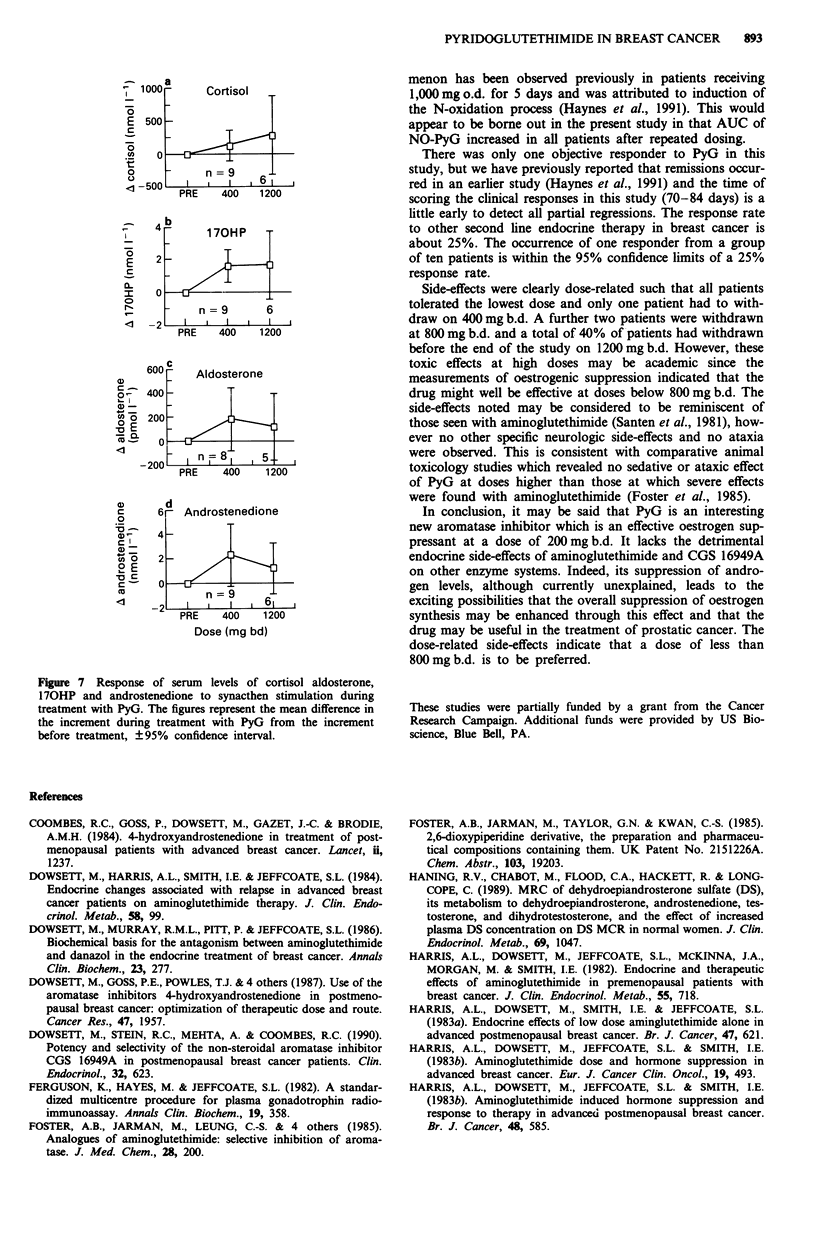
The aromatase inhibitor, 'pyridoglutethimide' (PyG), has been shown previously to suppress serum oestrogen levels in postmenopausal breast cancer patients and to achieve clinical responses at a dose of 500 mg twice daily (b.d.). This report gives the results of a detailed pharmacokinetic and endocrine study of PyG in ten patients. Four doses were tested at intervals of 2 weeks in the following order: 200 mg b.d., 400 mg b.d., 800 mg b.d., 1200 mg b.d. Concentration-time profiles of serum levels of PyG were curvilinear in all patients probably reflecting a saturation of metabolic enzymes. During repeat-dosing metabolism was enhanced approximately 2-fold. Plasma levels of oestradiol were significantly suppressed by the lowest dose of PyG. Although higher doses appeared to achieve greater suppression this was not statistically significant in this small group of patients. There were no significant effects at any dose on the serum levels of cortisol, aldosterone, luteinising hormone, follicle stimulating hormone, prolactin, sex hormone binding globulin or thyroid stimulating hormone. There was a dose-related increase in 17 alpha-hydroxyprogesterone levels and a dose-related decrease in levels of dehydroepiandrosterone sulphate (DHAS). The androgens DHA, testosterone and androstenedione also were significantly suppressed with at least one of the doses of PyG. Synacthen tests did not support these changes being a result of inhibition of 17,20 lyase. It is possible that they are due to enhanced clearance of DHAS. Two patients experienced no toxicity throughout the study, whilst a total of four patients were withdrawn because of side-effects: one at 400 mg b.d., two at 800 mg b.d., and one at 1200 mg b.d. The most frequent side-effects were nausea and lethargy. One patient showed an objective response to treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coombes R. C., Goss P., Dowsett M., Gazet J. C., Brodie A. 4-Hydroxyandrostenedione in treatment of postmenopausal patients with advanced breast cancer. Lancet. 1984 Dec 1;2(8414):1237–1239. doi: 10.1016/s0140-6736(84)92795-8. [DOI] [PubMed] [Google Scholar]
- Dowsett M., Goss P. E., Powles T. J., Hutchinson G., Brodie A. M., Jeffcoate S. L., Coombes R. C. Use of the aromatase inhibitor 4-hydroxyandrostenedione in postmenopausal breast cancer: optimization of therapeutic dose and route. Cancer Res. 1987 Apr 1;47(7):1957–1961. [PubMed] [Google Scholar]
- Dowsett M., Harris A. L., Smith I. E., Jeffcoate S. L. Endocrine changes associated with relapse in advanced breast cancer patients on aminoglutethimide therapy. J Clin Endocrinol Metab. 1984 Jan;58(1):99–104. doi: 10.1210/jcem-58-1-99. [DOI] [PubMed] [Google Scholar]
- Dowsett M., Murray R. M., Pitt P., Jeffcoate S. L. Biochemical basis for the antagonism between aminoglutethimide and danazol in the endocrine treatment of breast cancer. Ann Clin Biochem. 1986 May;23(Pt 3):277–284. doi: 10.1177/000456328602300306. [DOI] [PubMed] [Google Scholar]
- Dowsett M., Stein R. C., Mehta A., Coombes R. C. Potency and selectivity of the non-steroidal aromatase inhibitor CGS 16949A in postmenopausal breast cancer patients. Clin Endocrinol (Oxf) 1990 May;32(5):623–634. doi: 10.1111/j.1365-2265.1990.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Ferguson K. M., Hayes M., Jeffcoate S. L. A standardised multicentre procedure for plasma gonadotrophin radioimmunoassay. Ann Clin Biochem. 1982 Sep;19(Pt 5):358–361. doi: 10.1177/000456328201900507. [DOI] [PubMed] [Google Scholar]
- Foster A. B., Jarman M., Leung C. S., Rowlands M. G., Taylor G. N., Plevey R. G., Sampson P. Analogues of aminoglutethimide: selective inhibition of aromatase. J Med Chem. 1985 Feb;28(2):200–204. doi: 10.1021/jm00380a009. [DOI] [PubMed] [Google Scholar]
- Haning R. V., Jr, Chabot M., Flood C. A., Hackett R., Longcope C. Metabolic clearance rate (MCR) of dehydroepiandrosterone sulfate (DS), its metabolism to dehydroepiandrosterone, androstenedione, testosterone, and dihydrotestosterone, and the effect of increased plasma DS concentration on DS MCR in normal women. J Clin Endocrinol Metab. 1989 Nov;69(5):1047–1052. doi: 10.1210/jcem-69-5-1047. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Dowsett M., Jeffcoate S. L., McKinna J. A., Morgan M., Smith I. E. Endocrine and therapeutic effects of aminoglutethimide in premenopausal patients with breast cancer. J Clin Endocrinol Metab. 1982 Oct;55(4):718–722. doi: 10.1210/jcem-55-4-718. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Dowsett M., Jeffcoate S. L., Smith I. E. Aminoglutethimide dose and hormone suppression in advanced breast cancer. Eur J Cancer Clin Oncol. 1983 Apr;19(4):493–498. doi: 10.1016/0277-5379(83)90112-8. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Dowsett M., Smith I. E., Jeffcoate S. L. Endocrine effects of low dose aminoglutethimide alone in advanced postmenopausal breast cancer. Br J Cancer. 1983 May;47(5):621–627. doi: 10.1038/bjc.1983.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Dowsett M., Smith I. E., Jeffcoate S. Aminoglutethimide induced hormone suppression and response to therapy in advanced postmenopausal breast cancer. Br J Cancer. 1983 Oct;48(4):585–594. doi: 10.1038/bjc.1983.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. P., Jarman M., Dowsett M., Mehta A., Lønning P. E., Griggs L. J., Jones A., Powles T., Stein R., Coombes R. C. Pharmacokinetics and pharmacodynamics of the aromatase inhibitor 3-ethyl-3-(4-pyridyl)piperidine-2,6-dione in patients with postmenopausal breast cancer. Cancer Chemother Pharmacol. 1991;27(5):367–372. doi: 10.1007/BF00688859. [DOI] [PubMed] [Google Scholar]
- Lønning P. E., Johannessen D. C., Thorsen T. Alterations in the production rate and the metabolism of oestrone and oestrone sulphate in breast cancer patients treated with aminoglutethimide. Br J Cancer. 1989 Jul;60(1):107–111. doi: 10.1038/bjc.1989.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lønning P. E., Schanche J. S., Kvinnsland S., Ueland P. M. Single-dose and steady-state pharmacokinetics of aminoglutethimide. Clin Pharmacokinet. 1985 Jul-Aug;10(4):353–364. doi: 10.2165/00003088-198510040-00005. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Santner S., Davis B., Veldhuis J., Samojlik E., Ruby E. Aminoglutethimide inhibits extraglandular estrogen production in postmenopausal women with breast carcinoma. J Clin Endocrinol Metab. 1978 Dec;47(6):1257–1265. doi: 10.1210/jcem-47-6-1257. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Wells S. A., Cohn N., Demers L. M., Misbin R. I., Foltz E. L. Compensatory increase in TSH secretion without effect on prolactin secretion in patients treated with aminoglutethimide. J Clin Endocrinol Metab. 1977 Oct;45(4):739–746. doi: 10.1210/jcem-45-4-739. [DOI] [PubMed] [Google Scholar]
- Stein R. C., Dowsett M., Davenport J., Hedley A., Ford H. T., Gazet J. C., Coombes R. C. Preliminary study of the treatment of advanced breast cancer in postmenopausal women with the aromatase inhibitor CGS 16949A. Cancer Res. 1990 Mar 1;50(5):1381–1384. [PubMed] [Google Scholar]
- Stuart-Harris R., Dowsett M., D'Souza A., Donaldson A., Harris A. L., Jeffcoate S. L., Smith I. E. Endocrine effects of low dose aminoglutethimide as an aromatase inhibitor in the treatment of breast cancer. Clin Endocrinol (Oxf) 1985 Feb;22(2):219–226. doi: 10.1111/j.1365-2265.1985.tb01083.x. [DOI] [PubMed] [Google Scholar]


