Abstract
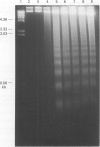
A number of reports indicate that protein synthesis is a requirement for the occurrence of apoptosis. In this study, the effect of the protein synthesis inhibitor cycloheximide (CHM) on spontaneous apoptosis of B-chronic lymphocytic leukaemia (B-CLL) cells, previously shown to occur when they are cultured in RPMI-1640 medium with autologous or heterologous serum, was examined. No definite inhibition of apoptosis was observed. Indeed, CHM-treatment augmented apoptosis in the B-CLL cultures and also induced apoptosis of cultured normal peripheral blood lymphocytes. Augmentation was dose-dependent for B-CLL cells over the concentration range 10(-6) M (0.28 micrograms ml-1) to 10(-2) M (2800 micrograms ml-1), resulting in 9% to 98% apoptosis respectively by 24 h of culture (r = 0.619, P = 0.0008). Normal lymphocytes were affected by CHM over the range 10(-4) M to 10(-2) M, resulting in 7% to 74% apoptosis respectively (r = 0.794, P = 0.0001). Inhibition of protein synthesis in these cells by CHM was virtually complete at a concentration of 10(-3) M. The findings are in accord with some recent reports indicating that suppression of protein synthesis by CHM does not inhibit apoptosis in all circumstances. They also illustrate the marked susceptibility of B-CLL cells, compared with normal lymphocytes, to the induction of apoptosis by this drug. The manner in which CHM triggers apoptosis of some cell types is at present uncertain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Shimada Y., Kaji K., Hayashi H. Apoptosis of vascular endothelial cells by fibroblast growth factor deprivation. Biochem Biophys Res Commun. 1990 May 16;168(3):1194–1200. doi: 10.1016/0006-291x(90)91155-l. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Bishop C. J., Moss D. J., Ryan J. M., Burrows S. R. T lymphocytes in infectious mononucleosis. II. Response in vitro to interleukin-2 and establishment of T cell lines. Clin Exp Immunol. 1985 Apr;60(1):70–77. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Chervenak R., Sellins K. S., Olson L. K. DNA fragmentation in targets of CTL: an example of programmed cell death in the immune system. Adv Exp Med Biol. 1985;184:493–508. doi: 10.1007/978-1-4684-8326-0_32. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Collins R. J., Verschuer L. A., Harmon B. V., Prentice R. L., Pope J. H., Kerr J. F. Spontaneous programmed death (apoptosis) of B-chronic lymphocytic leukaemia cells following their culture in vitro. Br J Haematol. 1989 Mar;71(3):343–350. doi: 10.1111/j.1365-2141.1989.tb04290.x. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder P. K., Schmidt L. J., Ono T., Getz M. J. Specific stimulation of actin gene transcription by epidermal growth factor and cycloheximide. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7476–7480. doi: 10.1073/pnas.81.23.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Rapid qualitative changes in mRNA populations in cultured human lymphocytes: comparison of the effects of cycloheximide and concanavalin A. Can J Biochem Cell Biol. 1984 Sep;62(9):859–864. doi: 10.1139/o84-110. [DOI] [PubMed] [Google Scholar]
- Galili U., Leizerowitz R., Moreb J., Gamliel H., Gurfel D., Polliack A. Metabolic and ultrastructural aspects of the in vitro lysis of chronic lymphocytic leukemia cells by glucocorticoids. Cancer Res. 1982 Apr;42(4):1433–1440. [PubMed] [Google Scholar]
- Ishihara T., Kudo A., Watanabe T. Induction of immunoglobulin gene expression in mouse fibroblasts by cycloheximide treatment. J Exp Med. 1984 Dec 1;160(6):1937–1942. doi: 10.1084/jem.160.6.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J., Dewey W. C. Protection of Chinese hamster ovary cells from hyperthermic killing by cycloheximide or puromycin. Radiat Res. 1986 Apr;106(1):98–110. [PubMed] [Google Scholar]
- Lieberman M. W., Verbin R. S., Landay M., Liang H., Farber E., Lee T. N., Starr R. A probable role for protein synthesis in intestinal epithelial cell damage induced in vivo by cytosine arabinoside, nitrogen mustard, or X-irradiation. Cancer Res. 1970 Apr;30(4):942–951. [PubMed] [Google Scholar]
- Makino R., Hayashi K., Sugimura T. C-myc transcript is induced in rat liver at a very early stage of regeneration or by cycloheximide treatment. Nature. 1984 Aug 23;310(5979):697–698. doi: 10.1038/310697a0. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Lennon S. V., Bonham A. M., Cotter T. G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990 Sep 15;145(6):1859–1867. [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Duddy S. K., Håkansson H., Orrenius S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science. 1988 Oct 14;242(4876):256–259. doi: 10.1126/science.3262923. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Mak S. Actinomycin D-induced breakage of human KB cell DNA. Nature. 1974 Aug 30;250(5469):786–788. doi: 10.1038/250786a0. [DOI] [PubMed] [Google Scholar]
- Portugal J., Waring M. J. Analysis of the effects of antibiotics on the structure of nucleosome core particles determined by DNAase I cleavage. Biochimie. 1987 Aug;69(8):825–840. doi: 10.1016/0300-9084(87)90209-4. [DOI] [PubMed] [Google Scholar]
- Pratt R. M., Greene R. M. Inhibition of palatal epithelial cell death by altered protein synthesis. Dev Biol. 1976 Nov;54(1):135–145. doi: 10.1016/0012-1606(76)90292-x. [DOI] [PubMed] [Google Scholar]
- Searle J., Lawson T. A., Abbott P. J., Harmon B., Kerr J. F. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol. 1975 Jul;116(3):129–138. doi: 10.1002/path.1711160302. [DOI] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
- Tata J. R. Requirement for RNA and protein synthesis for induced regression of the tadpole tail in organ culture. Dev Biol. 1966 Feb;13(1):77–94. doi: 10.1016/0012-1606(66)90050-9. [DOI] [PubMed] [Google Scholar]
- Walker N. I., Harmon B. V., Gobé G. C., Kerr J. F. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- Waring P. DNA fragmentation induced in macrophages by gliotoxin does not require protein synthesis and is preceded by raised inositol triphosphate levels. J Biol Chem. 1990 Aug 25;265(24):14476–14480. [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Yamada T., Ohyama H. Radiation-induced interphase death of rat thymocytes is internally programmed (apoptosis). Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Jan;53(1):65–75. doi: 10.1080/09553008814550431. [DOI] [PubMed] [Google Scholar]
- Zola H. Fractionation of human lymphocytes using rosette formation with papain-treated mouse erythrocytes. J Immunol Methods. 1977;18(3-4):387–389. doi: 10.1016/0022-1759(77)90193-4. [DOI] [PubMed] [Google Scholar]