Abstract
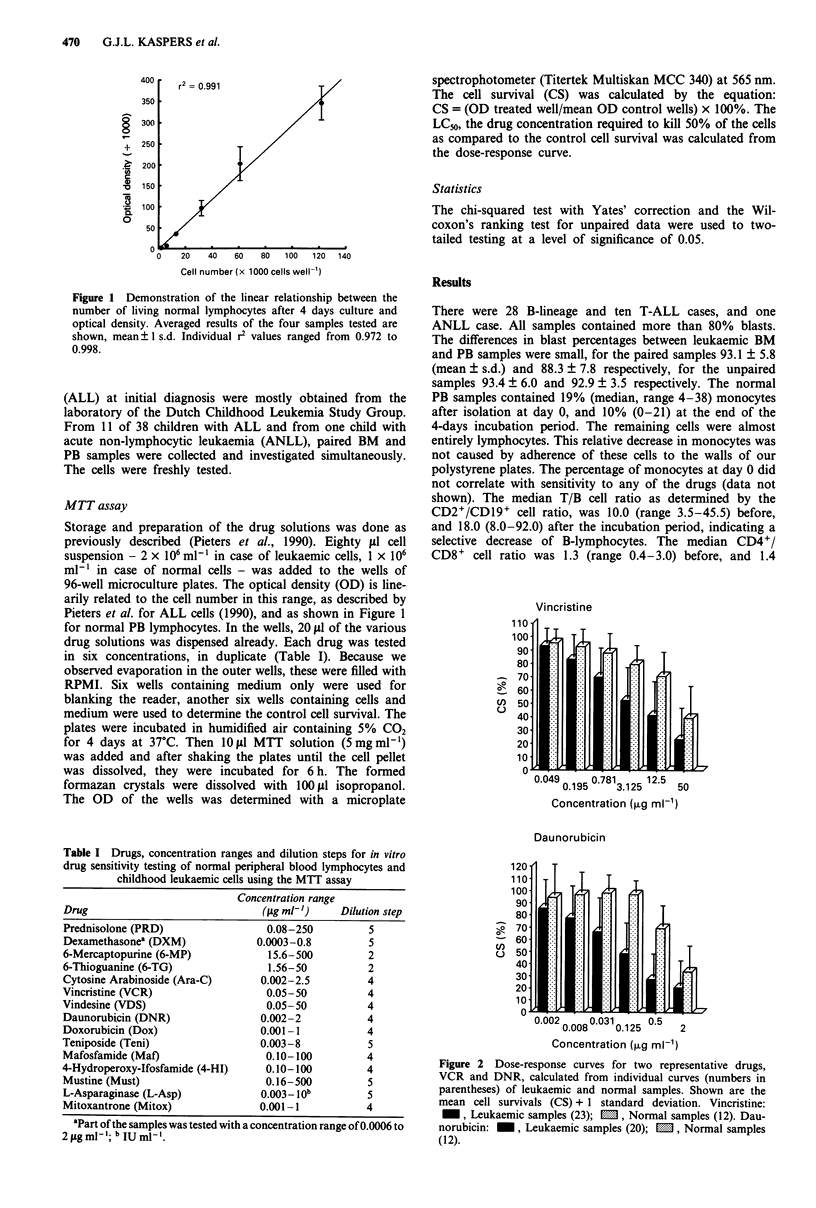
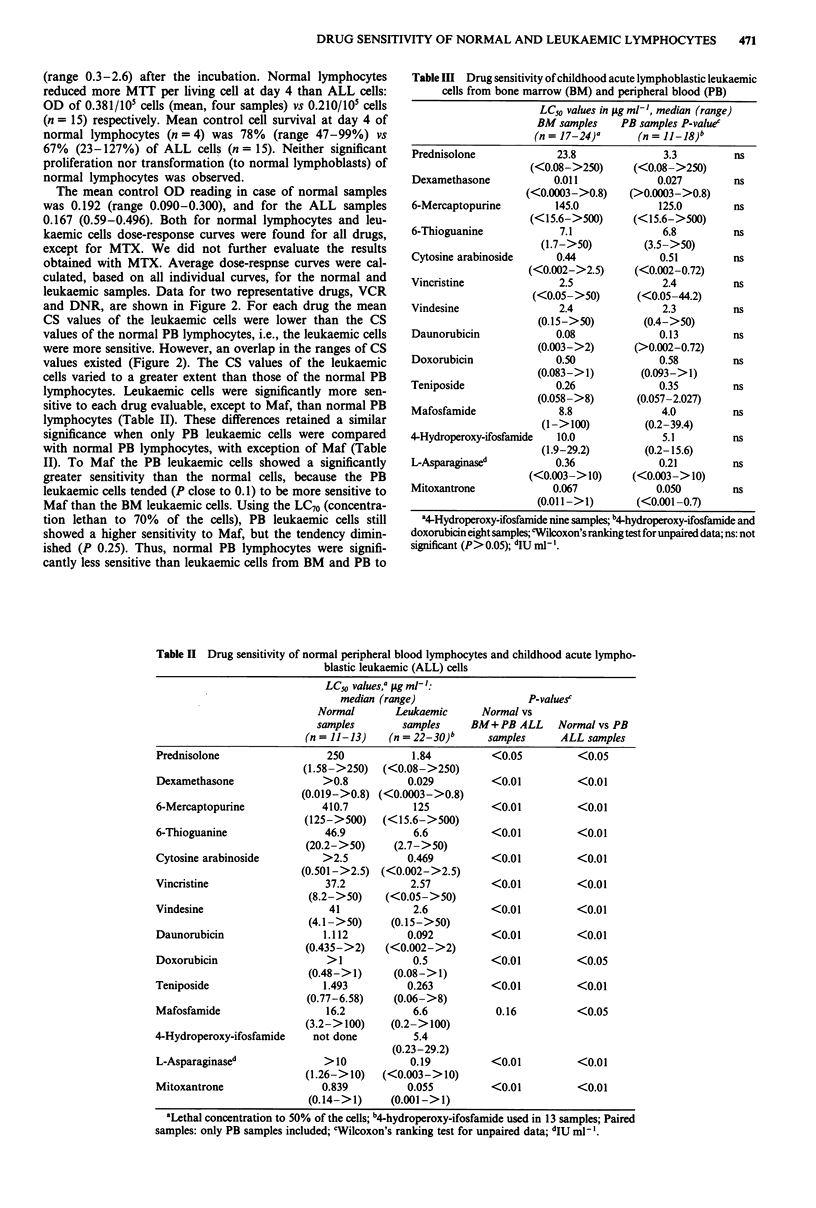
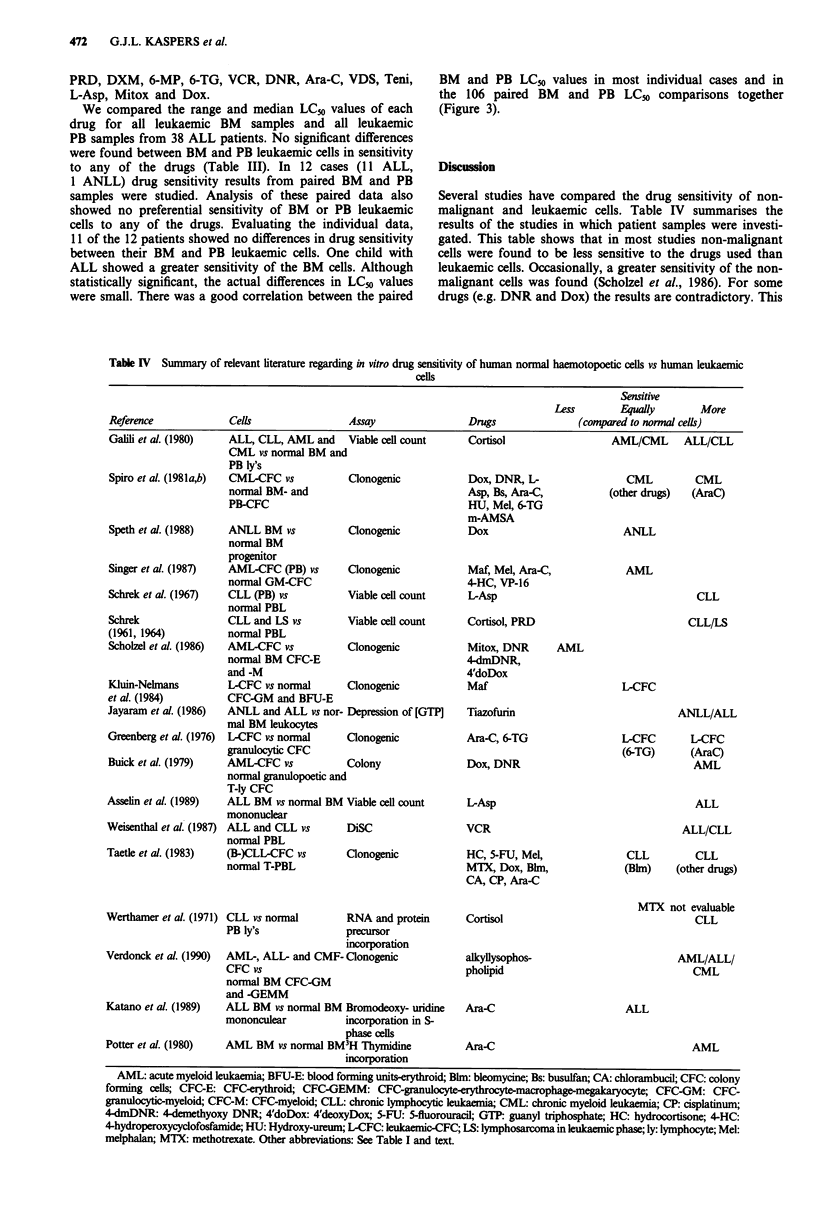
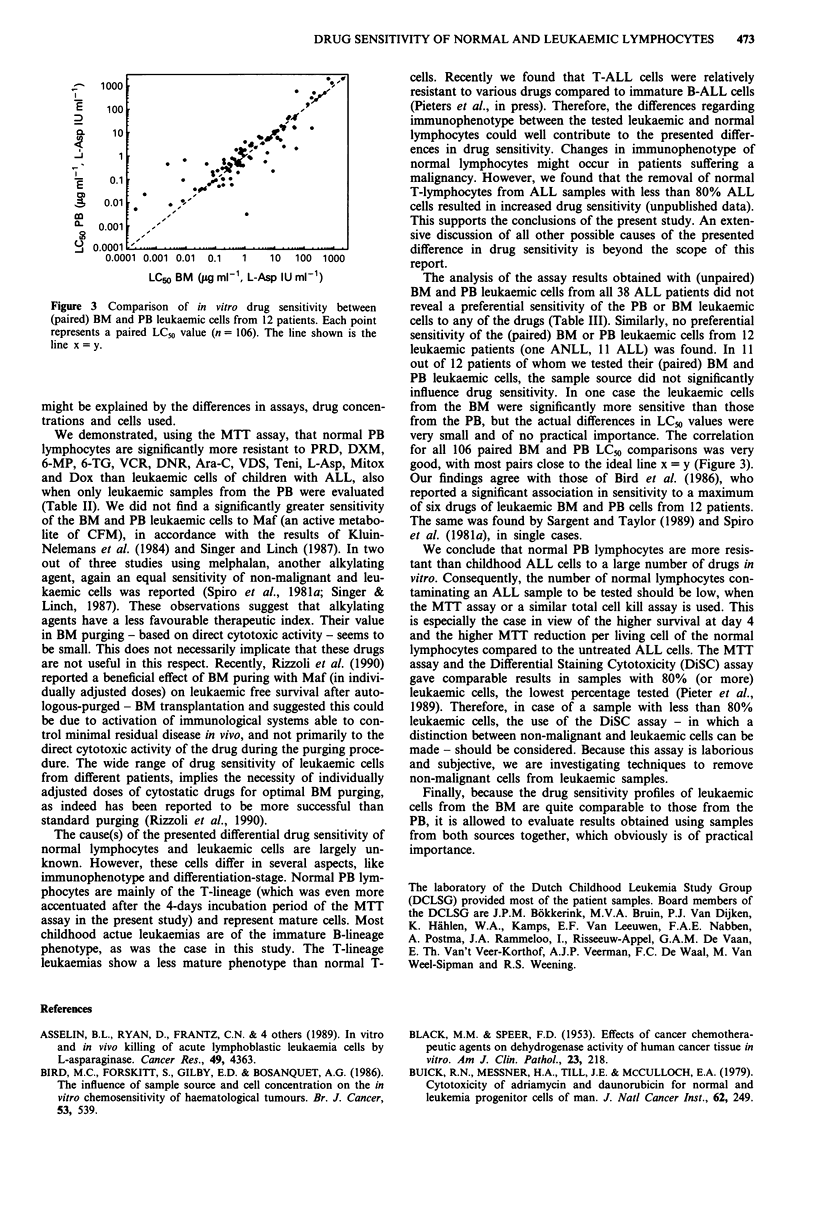
In vitro drug sensitivity of leukaemic cells might be influenced by the contamination of such a sample with non-malignant cells and the sample source. To study this, sensitivity of normal peripheral blood (PB) lymphocytes to a number of cytostatic drugs was assessed with the MTT assay. We compared this sensitivity with the drug sensitivity of leukaemic cells of 38 children with acute lymphoblastic leukaemia. We also studied a possible differential sensitivity of leukaemic cells from bone marrow (BM) and PB. The following drugs were used: Prednisolone, dexamethasone, 6-mercaptopurine, 6-thioguanine, cytosine arabinoside, vincristine, vindesine, daunorubicin, doxorubicin, mafosfamide (Maf), 4-hydroperoxy-ifosfamide, teniposide, mitoxantrone, L-asparaginase, methotrexate and mustine. Normal PB lymphocytes were significantly more resistant to all drugs tested, except to Maf. Leukaemic BM and PB cells from 38 patients (unpaired samples) showed no significant differences in sensitivity to any of the drugs. Moreover, in 11 of 12 children with acute leukaemia of whom we investigated simultaneously obtained BM and PB (paired samples), their leukaemic BM and PB cells showed comparable drug sensitivity profiles. In one patient the BM cells were more sensitive to most drugs than those from the PB, but the actual differences in sensitivity were small. We conclude that the contamination of a leukaemic sample with normal PB lymphocytes will influence the results of the MTT assay. The source of the leukaemic sample, BM or PB, does not significantly influence the assay results.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselin B. L., Ryan D., Frantz C. N., Bernal S. D., Leavitt P., Sallan S. E., Cohen H. J. In vitro and in vivo killing of acute lymphoblastic leukemia cells by L-asparaginase. Cancer Res. 1989 Aug 1;49(15):4363–4368. [PubMed] [Google Scholar]
- BLACK M. M., SPEER F. D. Effects of cancer chemotherapeutic agents on dehydrogenase activity of human cancer tissue in vitro. Am J Clin Pathol. 1953 Mar;23(3):218–227. doi: 10.1093/ajcp/23.3.218. [DOI] [PubMed] [Google Scholar]
- Bird M. C., Forskitt S., Gilby E. D., Bosanquet A. G. The influence of sample source and cell concentration on the in vitro chemosensitivity of haematological tumours. Br J Cancer. 1986 Apr;53(4):539–545. doi: 10.1038/bjc.1986.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buick R. N., Messner H. A., Till J. E., McCulloch E. A. Cytotoxicity of adriamycin and daunorubicin for normal and leukemia progenitor cells of man. J Natl Cancer Inst. 1979 Feb;62(2):249–255. [PubMed] [Google Scholar]
- Galili U., Prokocimer M., Izak G. The in vitro sensitivity of leukemic and normal leukocytes to hydrocortisone induced cytolysis. Blood. 1980 Dec;56(6):1077–1081. [PubMed] [Google Scholar]
- Greenberg P. L., VanKersen I., Mosny S. Cytotoxic effects of 1-beta-D-arabinofuranosylcytosine and 6-thioguanine in vitro on granulocytic progenitor cells. Cancer Res. 1976 Dec;36(12):4412–4417. [PubMed] [Google Scholar]
- Jayaram H. N., Pillwein K., Nichols C. R., Hoffman R., Weber G. Selective sensitivity to tiazofurin of human leukemic cells. Biochem Pharmacol. 1986 Jun 15;35(12):2029–2032. doi: 10.1016/0006-2952(86)90737-9. [DOI] [PubMed] [Google Scholar]
- Katano N., Tsurusawa M., Niwa M., Fujimoto T. Flow cytometric determination with bromodeoxyuridine/DNA assay of sensitivity of S-phase cells to cytosine arabinoside in childhood acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1989 Winter;11(4):411–416. [PubMed] [Google Scholar]
- Kirkpatrick D. L., Duke M., Goh T. S. Chemosensitivity testing of fresh human leukemia cells using both a dye exclusion assay and a tetrazolium dye (MTT) assay. Leuk Res. 1990;14(5):459–466. doi: 10.1016/0145-2126(90)90033-6. [DOI] [PubMed] [Google Scholar]
- Kluin-Nelemans H. C., Martens A. C., Löwenberg B., Hagenbeek A. No preferential sensitivity of clonogenic AML cells to ASTA-Z-7557. Leuk Res. 1984;8(4):723–728. doi: 10.1016/0145-2126(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Pieters R., Huismans D. R., Leyva A., Veerman A. J. Adaptation of the rapid automated tetrazolium dye based (MTT) assay for chemosensitivity testing in childhood leukemia. Cancer Lett. 1988 Aug 30;41(3):323–332. doi: 10.1016/0304-3835(88)90294-7. [DOI] [PubMed] [Google Scholar]
- Pieters R., Huismans D. R., Leyva A., Veerman A. J. Comparison of the rapid automated MTT-assay with a dye exclusion assay for chemosensitivity testing in childhood leukaemia. Br J Cancer. 1989 Feb;59(2):217–220. doi: 10.1038/bjc.1989.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters R., Loonen A. H., Huismans D. R., Broekema G. J., Dirven M. W., Heyenbrok M. W., Hählen K., Veerman A. J. In vitro drug sensitivity of cells from children with leukemia using the MTT assay with improved culture conditions. Blood. 1990 Dec 1;76(11):2327–2336. [PubMed] [Google Scholar]
- Potter C. G., Bunch C. Sensitivity of normal and acute myelogenous leukaemia marrow cells to inhibition by cytosine arabinoside. Br J Cancer. 1980 Jun;41(6):985–988. doi: 10.1038/bjc.1980.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli V., Carella A. M., Carlo-Stella C., Mangoni L. Autologous marrow transplantation in acute lymphoblastic leukemia: control of residual disease with mafosfamide and induction of syngeneic GVHD with cyclosporin. The Italian Mafosfamide Study Group. Bone Marrow Transplant. 1990 Jul;6 (Suppl 1):76–78. [PubMed] [Google Scholar]
- SCHREK R. Cytotoxicity of adrenal cortex hormones on normal and malignant lymphocytes of man and rat. Proc Soc Exp Biol Med. 1961 Nov;108:328–332. doi: 10.3181/00379727-108-26928. [DOI] [PubMed] [Google Scholar]
- SCHREK R. PREDNISOLONE SENSITIVITY AND CYTOLOGY OF VIABLE LYMPHOCYTES AS TESTS FOR CHRONIC LYMPHOCYTIC LEUKEMIA. J Natl Cancer Inst. 1964 Nov;33:837–847. doi: 10.1093/jnci/33.5.837. [DOI] [PubMed] [Google Scholar]
- Schrek R., Dolowy W. C., Ammeraal R. N. L-asparaginase: toxicity to normal and leukemic human lymphocytes. Science. 1967 Jan 20;155(3760):329–330. doi: 10.1126/science.155.3760.329. [DOI] [PubMed] [Google Scholar]
- Schölzel C., van Putten W., Löwenberg B. A comparison of in vitro sensitivity of acute myeloid leukemia precursors to mitoxantrone, 4'deoxydoxorubicin, 4-demethoxydaunorubicin and daunorubicin. Leuk Res. 1986;10(12):1455–1459. doi: 10.1016/0145-2126(86)90013-5. [DOI] [PubMed] [Google Scholar]
- Singer C. R., Linch D. C. Comparison of the sensitivity of normal and leukaemic myeloid progenitors to in-vitro incubation with cytotoxic drugs: a study of pharmacological purging. Leuk Res. 1987;11(11):953–959. doi: 10.1016/0145-2126(87)90112-3. [DOI] [PubMed] [Google Scholar]
- Speth P. A., Raijmakers R. A., Boezeman J. B., Linssen P. C., de Witte T. J., Wessels H. M., Haanen C. In vivo cellular adriamycin concentrations related to growth inhibition of normal and leukemic human bone marrow cells. Eur J Cancer Clin Oncol. 1988 Apr;24(4):667–674. doi: 10.1016/0277-5379(88)90297-0. [DOI] [PubMed] [Google Scholar]
- Spiro T. E., Mattelaer M. A., Efira A., Stryckmans P. Sensitivity of myeloid progenitor cells in healthy subjects and patients with chronic myeloid leukemia to chemotherapeutic agents. J Natl Cancer Inst. 1981 Jun;66(6):1053–1059. doi: 10.1093/jnci/66.6.1053. [DOI] [PubMed] [Google Scholar]
- Spiro T. E., Socquet M., Delforge A., Stryckmans P. Chemotherapeutic sensitivity of normal and leukemic hematopoietic progenitor cells to N-[4-(9-acridinylamino)-3-methoxyphenyl]-methanesulfonamide, a new anticancer agent. J Natl Cancer Inst. 1981 Apr;66(4):615–618. [PubMed] [Google Scholar]
- Taetle R., To D., Mendelsohn J. In vitro sensitivity to steroid hormones and cytotoxic agents of normal and malignant lymphocyte colony-forming cells. Cancer Res. 1983 Aug;43(8):3553–3558. [PubMed] [Google Scholar]
- Veerman A. J., Pieters R. Drug sensitivity assays in leukaemia and lymphoma. Br J Haematol. 1990 Apr;74(4):381–384. doi: 10.1111/j.1365-2141.1990.tb06323.x. [DOI] [PubMed] [Google Scholar]
- Verdonck L. F., Witteveen E. O., van Heugten H. G., Rozemuller E., Rijksen G. Selective killing of malignant cells from leukemic patients by alkyl-lysophospholipid. Cancer Res. 1990 Jul 1;50(13):4020–4025. [PubMed] [Google Scholar]
- Weisenthal L. M., Su Y. Z., Duarte T. E., Dill P. L., Nagourney R. A. Perturbation of in vitro drug resistance in human lymphatic neoplasms by combinations of putative inhibitors of protein kinase C. Cancer Treat Rep. 1987 Dec;71(12):1239–1243. [PubMed] [Google Scholar]
- Werthamer S., Amaral L. The response of leukemic lymphocytes to cortisol: a suggested role of transcortin. Blood. 1971 Apr;37(4):463–472. [PubMed] [Google Scholar]


