Abstract
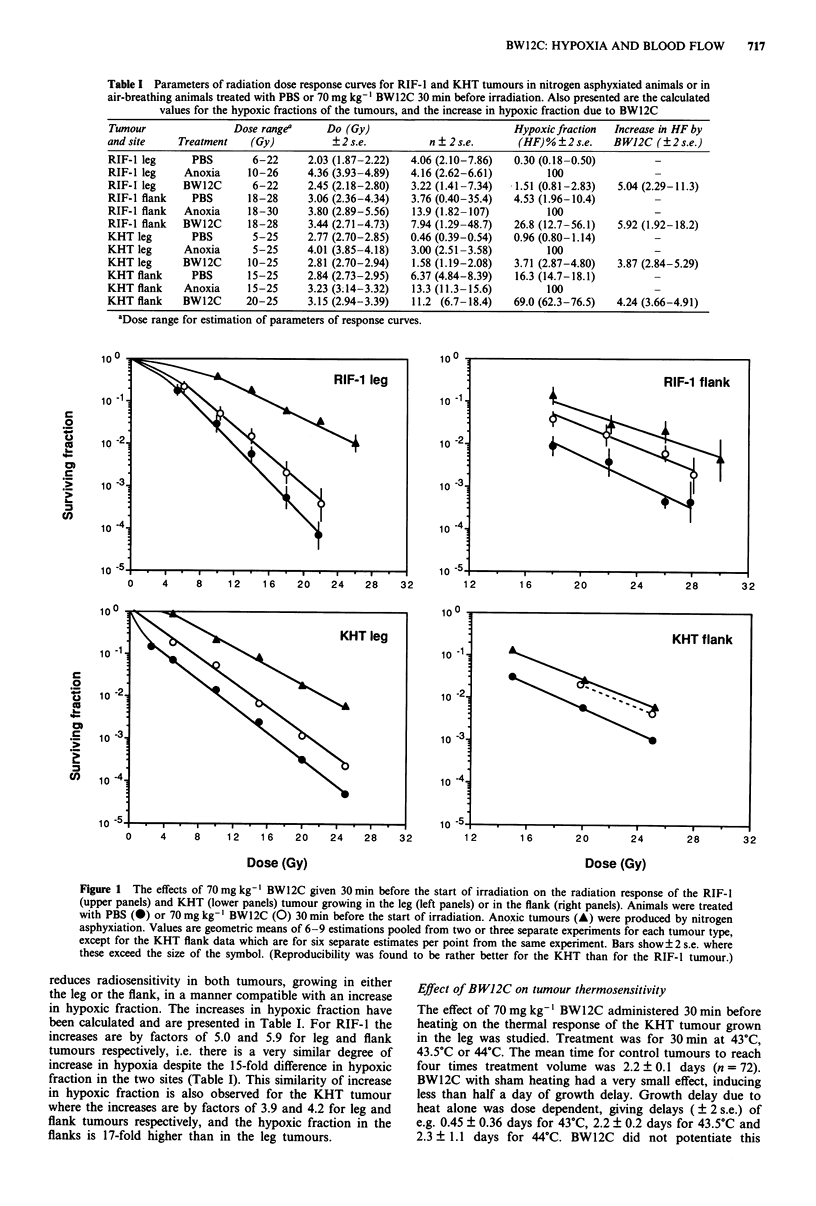
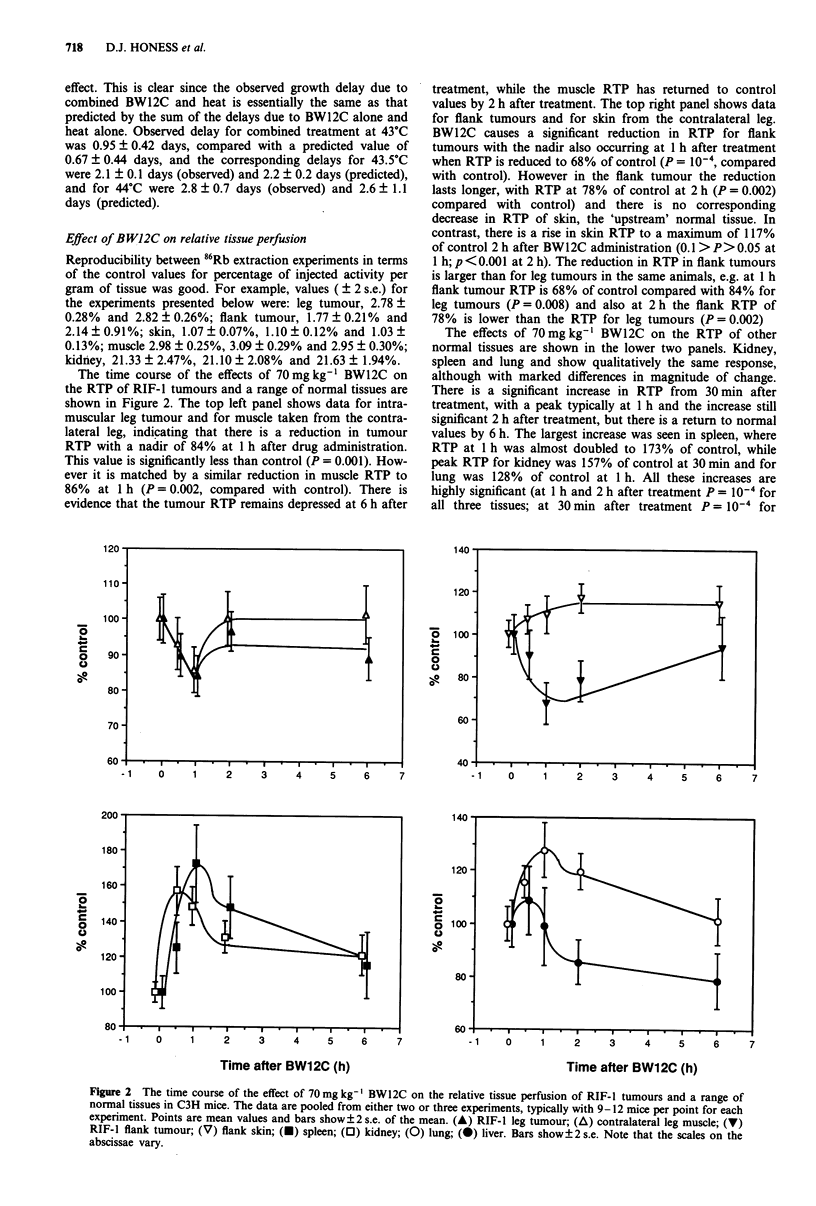
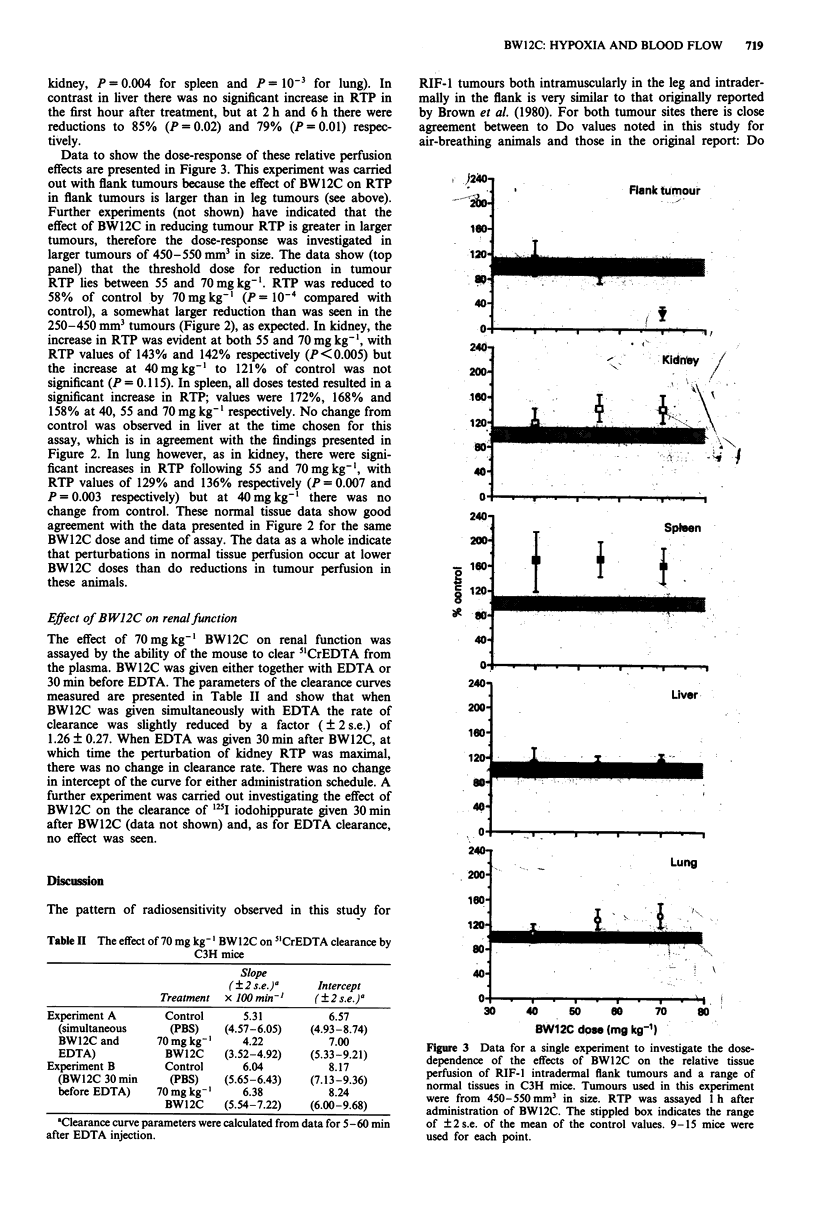
BW12C (5-[2-formyl-3-hydroxypenoxyl] pentanoic acid) is an agent which stabilises oxyhaemoglobin and thus reduces oxygen delivery to tissues. It is of interest as a possible potentiator of bioreductive agents and/or hyperthermia. The increases in radiobiological hypoxic fraction of RIF-1 and KHT tumours 30 min after 70 mg kg-1 BW12C i.v. were measured and shown to be similar; factors (+/- 2 s.e.) ranged from 3.87 (2.84-5.29) to 5.92 (1.92-18.2) despite the large variation in initial hypoxic fraction, from 0.30 (0.18-0.50) % for RIF-1 intramuscularly in the leg to 16.3 (14.7-18.1) % for subcutaneous KHT flank tumours. Thermosensitivity of intramuscular KHT leg tumours was not enhanced by 70 mg kg-1 BW12C 30 min before heating at 43 degrees C, 43.5 degrees C or 44 degrees C, assayed by regrowth delay. The effect of 70 mg kg-1 BW12C on relative tissue perfusion (RTP), assayed by 86Rb extraction, was measured from 0.5 h to 6 h after treatment. After 1 h RTP (+/- 2 s.e.) in RIF-1 tumours was reduced to 84 +/- 5.7% and 68 +/- 9.6% of control in leg and flank tumours respectively, and to 86 +/- 6.4% in leg muscle while flank skin RTP was unaltered at 109 +/- 8.6%. There were substantial increases in kidney (149 +/- 10.7%) spleen (173 +/- 22.1%) and lung (128 +/- 10.4%) at 1 h but in liver there was a decrease at 2 h to 85 +/- 8.4%. Dose response studies showed that the threshold dose for reduction of tumour RTP is between 55 and 70 mg kg-1, but perturbations in normal tissue RTP occur at lower doses, e.g. 40 mg kg-1 for spleen. BW12C had minimal effects on renal function measured by 51CrEDTA clearance. The data as a whole indicate that reduction in tumour perfusion is likely to be an important determinant in the increase in tumour hypoxia induced by BW12C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Barnes D. W., du Boulay C., Loutit J. F., Cole S., Sheldon P. W., Stratford I. J., van den Aardweg G. J., Hopewell J. W., White R. D. Induction of hypoxia in normal and malignant tissues by changing the oxygen affinity of hemoglobin--implications for therapy. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1299–1302. doi: 10.1016/0360-3016(86)90158-6. [DOI] [PubMed] [Google Scholar]
- Adams G. E., Stratford I. J., Nethersell A. B., White R. D. Induction of severe tumor hypoxia by modifiers of the oxygen affinity of hemoglobin. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1179–1182. doi: 10.1016/0360-3016(89)90278-2. [DOI] [PubMed] [Google Scholar]
- Beddell C. R., Goodford P. J., Kneen G., White R. D., Wilkinson S., Wootton R. Substituted benzaldehydes designed to increase the oxygen affinity of human haemoglobin and inhibit the sickling of sickle erythrocytes. Br J Pharmacol. 1984 Jun;82(2):397–407. doi: 10.1111/j.1476-5381.1984.tb10775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Twentyman P. R., Zamvil S. S. Response to the RIF-1 tumor in vitro and in C3H/Km mice to X-radiation (cell survival, regrowth delay, and tumor control), chemotherapeutic agents, and activated macrophages. J Natl Cancer Inst. 1980 Mar;64(3):605–611. [PubMed] [Google Scholar]
- Cole S., Robbins L. Manipulation of oxygenation in a human tumour xenograft with BW12C or hydralazine: effects on responses to radiation and to the bioreductive cytotoxicity of misonidazole or RSU-1069. Radiother Oncol. 1989 Nov;16(3):235–243. doi: 10.1016/0167-8140(89)90023-6. [DOI] [PubMed] [Google Scholar]
- Fitzharris P., McLean A. E., Sparks R. G., Weatherley B. C., White R. D., Wootton R. The effects in volunteers of BW12C, a compound designed to left-shift the blood-oxygen saturation curve. Br J Clin Pharmacol. 1985 Apr;19(4):471–481. doi: 10.1111/j.1365-2125.1985.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerweck L. E., Nygaard T. G., Burlett M. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Res. 1979 Mar;39(3):966–972. [PubMed] [Google Scholar]
- Hahn G. M. Metabolic aspects of the role of hyperthermia im mammalian cell inactivation and their possible relevance to cancer treatment. Cancer Res. 1974 Nov;34(11):3117–3123. [PubMed] [Google Scholar]
- Hill R. P. An appraisal of in vivo assays of excised tumours. Br J Cancer Suppl. 1980 Apr;4:230–239. [PMC free article] [PubMed] [Google Scholar]
- Honess D. J., Bleehen N. M. Sensitivity of normal mouse marrow and RIF-1 tumour to hyperthermia combined with cyclophosphamide or BCNU: a lack of therapeutic gain. Br J Cancer. 1982 Aug;46(2):236–248. doi: 10.1038/bjc.1982.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess D. J., Hu D. E., Bleehen N. M. A study of the mechanism of hydralazine enhancement of thermal damage in the KHT tumour. Int J Hyperthermia. 1991 Jul-Aug;7(4):667–679. doi: 10.3109/02656739109034978. [DOI] [PubMed] [Google Scholar]
- Honess D. J., White R. D., Nethersell A. B., Bleehen N. M. Effect of the manipulation of oxyhemoglobin status by BW12C on tumor thermosensitivity and on blood flow in tumor and normal tissues in mice. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1187–1190. doi: 10.1016/0360-3016(89)90280-0. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Christensen K. L., Overgaard J. Hydralazine-induced enhancement of hyperthermic damage in a C3H mammary carcinoma in vivo. Int J Hyperthermia. 1989 Mar-Apr;5(2):123–136. doi: 10.3109/02656738909140442. [DOI] [PubMed] [Google Scholar]
- Keidan A. J., Franklin I. M., White R. D., Joy M., Huehns E. R., Stuart J. Effect of BW12C on oxygen affinity of haemoglobin in sickle-cell disease. Lancet. 1986 Apr 12;1(8485):831–834. doi: 10.1016/s0140-6736(86)90941-4. [DOI] [PubMed] [Google Scholar]
- Kennedy K. A. Hypoxic cells as specific drug targets for chemotherapy. Anticancer Drug Des. 1987 Oct;2(2):181–194. [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Overgaard J., Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology. 1977 May;123(2):511–514. doi: 10.1148/123.2.511. [DOI] [PubMed] [Google Scholar]
- Overgaard J., Nielsen O. S. The role of tissue environmental factors on the kinetics and morphology of tumor cells exposed to hyperthermia. Ann N Y Acad Sci. 1980;335:254–280. doi: 10.1111/j.1749-6632.1980.tb50753.x. [DOI] [PubMed] [Google Scholar]
- SAPIRSTEIN L. A. Regional blood flow by fractional distribution of indicators. Am J Physiol. 1958 Apr;193(1):161–168. doi: 10.1152/ajplegacy.1958.193.1.161. [DOI] [PubMed] [Google Scholar]
- Sartorelli A. C. Therapeutic attack of hypoxic cells of solid tumors: presidential address. Cancer Res. 1988 Feb 15;48(4):775–778. [PubMed] [Google Scholar]
- Siemann D. W., Alliet K. L., Macler L. M. Manipulations in the oxygen transport capacity of blood as a means of sensitizing tumors to radiation therapy. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1169–1172. doi: 10.1016/0360-3016(89)90276-9. [DOI] [PubMed] [Google Scholar]
- Siemann D. W., Hill R. P., Bush R. S. Smoking: the influence of carboxyhemoglobin (HbCO) on tumor oxygenation and response to radiation. Int J Radiat Oncol Biol Phys. 1978 Jul-Aug;4(7-8):657–662. doi: 10.1016/0360-3016(78)90189-x. [DOI] [PubMed] [Google Scholar]
- Siemann D. W., Keng P. C. Characterization of radiation resistant hypoxic cell subpopulations in KHT sarcomas. (I). Centrifugal elutriation. Br J Cancer. 1987 Jan;55(1):33–36. doi: 10.1038/bjc.1987.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann D. W., Macler L. M. Tumor radiosensitization through reductions in hemoglobin affinity. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1295–1297. doi: 10.1016/0360-3016(86)90157-4. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Walton M. I., Cattermole D. M., Bleehen N. M. A microcomputer controlled, local hyperthermia system for uniform tumour heating in unanaesthetized mice. Int J Hyperthermia. 1989 Jan-Feb;5(1):53–58. doi: 10.3109/02656738909140432. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M., Lemmon M. J., Hirst V. K., Lee W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- van den Aardweg G. J., Hopewell J. W., Adams G. E., Barnes D. W., Sansom J. M., Stratford I. J., Nethersell A. B. Protection of pig epidermis against radiation-induced damage by the infusion of BW12C. Int J Radiat Biol. 1991 Apr;59(4):1039–1051. doi: 10.1080/09553009114550921. [DOI] [PubMed] [Google Scholar]


