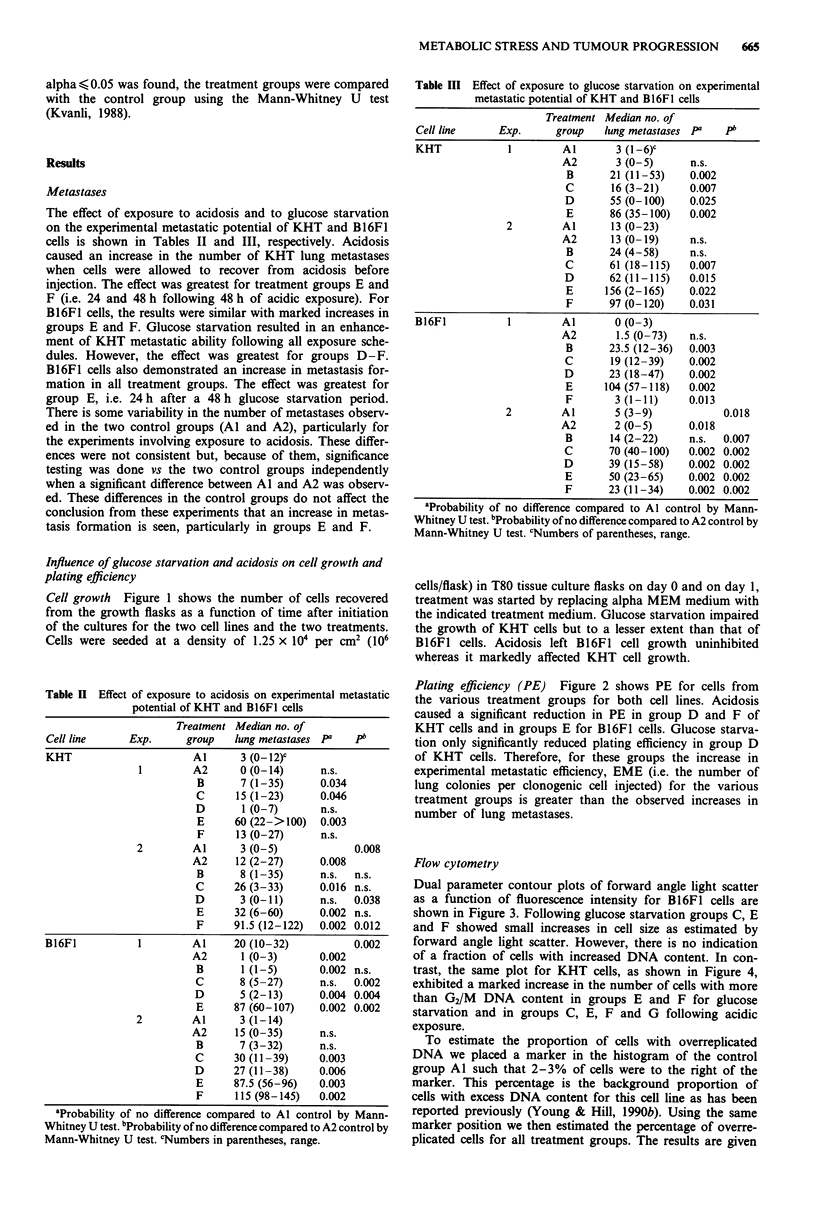
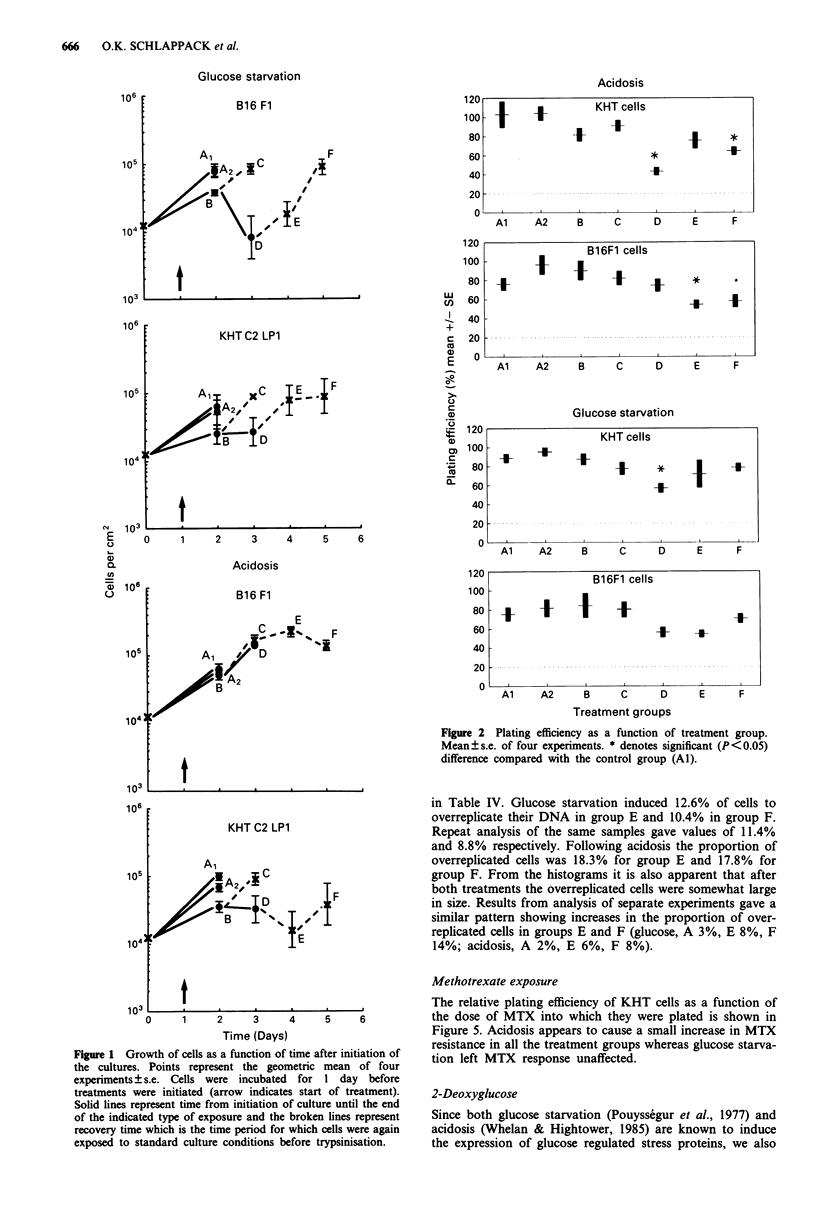
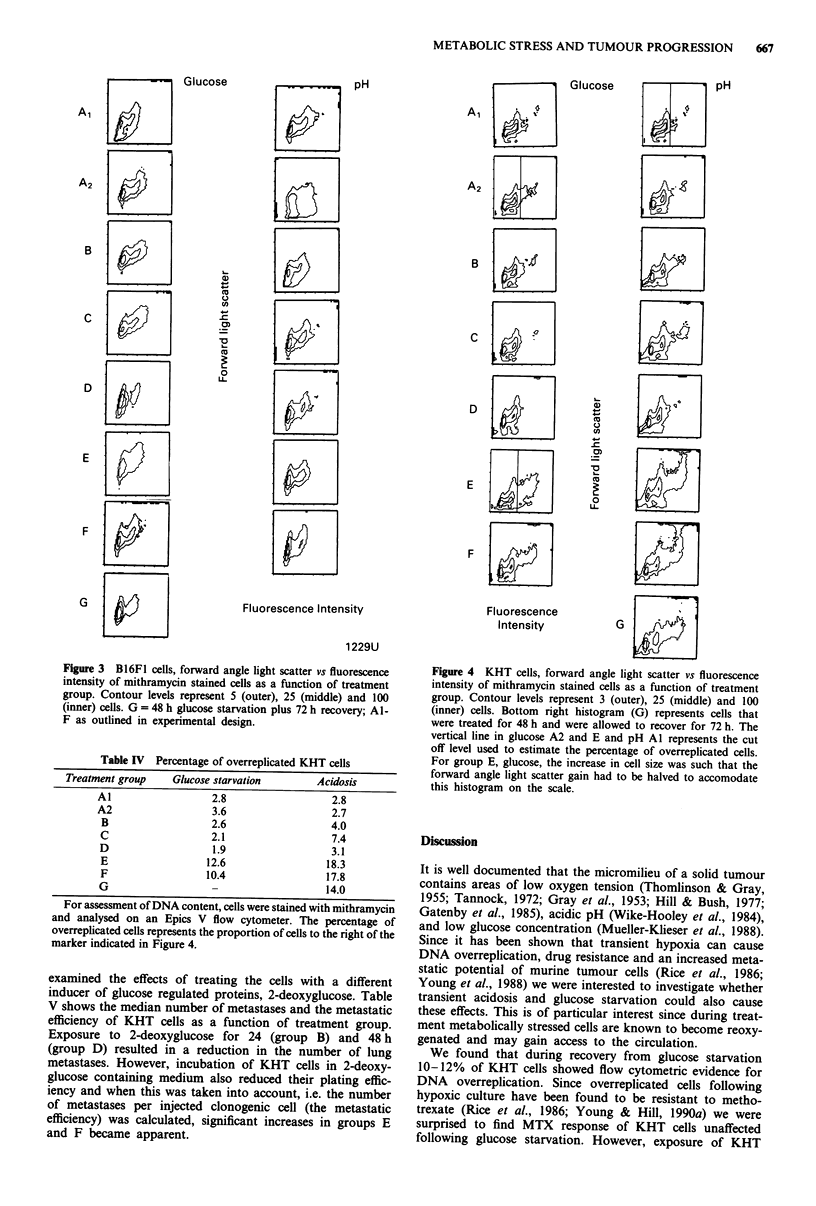
Abstract
Exposure to oxygen deprivation in vitro has been reported to cause drug resistance in CHO cells (Rice et al., 1986; PNAS 83, 5978) and enhancement of experimental metastatic (colonisation) ability of murine tumour cells (Young et al., 1988; PNAS 85, 9533). Both these studies also demonstrated the induction of a subpopulation of cells with excess DNA content. Since the micromilieu in tumours results in exposure of the tumour cells to conditions of acid pH and nutrient deprivation, as well as hypoxia, we have examined the effect of exposure to acidosis (pH 6.5) and glucose starvation on drug resistance, cellular DNA content and the experimental metastatic ability of KHT sarcoma and B16F1 melanoma cells. Cells were exposed to these conditions for 24 and 48 h and tested for resistance to methotrexate (MTX) or experimental metastatic ability either immediately following these exposures or after 24 or 48 h of recovery in normal growth medium. Both cell lines demonstrated an enhancement of colonisation potential, which was most marked when cells were injected after 48 h of exposure followed by a 24 or 48 h recovery period. Flow cytometric analysis demonstrated an increase in the fraction of KHT cells with excess DNA following both glucose starvation and acidosis we observed only a small increase in MTX resistance following acidic exposure of cells and no change following glucose starvation. Since both acidosis and glucose starvation are known to induce glucose regulated proteins (grp), a subset of the stress protein family, we studied the effect of treatment with another known inducer, 2-deoxyglucose. We found that this agent affected the metastatic efficiency of KHT cells in a manner similar to that observed following exposure to glucose starvation and acidosis. However, further studies are required to establish what role, if any, grp play in this effect. In conclusion this study shows that transient exposure of murine tumour cells to an acidic or glucose deprived environment can cause progression in terms of metastatic potential.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Stoler D. L., Scarcello L. A. Normal fibroblasts responding to anoxia exhibit features of the malignant phenotype. J Biol Chem. 1989 Sep 5;264(25):14885–14892. [PubMed] [Google Scholar]
- Armitage N. C., Robins R. A., Evans D. F., Turner D. R., Baldwin R. W., Hardcastle J. D. The influence of tumour cell DNA abnormalities on survival in colorectal cancer. Br J Surg. 1985 Oct;72(10):828–830. doi: 10.1002/bjs.1800721018. [DOI] [PubMed] [Google Scholar]
- Born R., Eichholtz-Wirth H. Effect of different physiological conditions on the action of adriamycin on Chinese hamster cells in vitro. Br J Cancer. 1981 Aug;44(2):241–246. doi: 10.1038/bjc.1981.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh P. G., Nicolson G. L. Purification and characterization of a Mr approximately 66,000 lung-derived (paracrine) growth factor that preferentially stimulates the in vitro proliferation of lung-metastasizing tumor cells. J Cell Biochem. 1990 Jun;43(2):127–138. doi: 10.1002/jcb.240430204. [DOI] [PubMed] [Google Scholar]
- Cavanaugh P. G., Nicolson G. L. Purification and some properties of a lung-derived growth factor that differentially stimulates the growth of tumor cells metastatic to the lung. Cancer Res. 1989 Jul 15;49(14):3928–3933. [PubMed] [Google Scholar]
- Cooke L. D., Cooke T. G., Bootz F., Forster G., Helliwell T. R., Spiller D., Stell P. M. Ploidy as a prognostic indicator in end stage squamous cell carcinoma of the head and neck region treated with cisplatinum. Br J Cancer. 1990 May;61(5):759–762. doi: 10.1038/bjc.1990.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelisse C. J., van de Velde C. J., Caspers R. J., Moolenaar A. J., Hermans J. DNA ploidy and survival in breast cancer patients. Cytometry. 1987 Mar;8(2):225–234. doi: 10.1002/cyto.990080217. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Tobey R. A. Cell-cycle analysis in 20 minutes. Science. 1974 Jun 21;184(4143):1297–1298. doi: 10.1126/science.184.4143.1297. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Greenberg A. H., Egan S. E., Hamilton R. T., Wright J. A. Cysteine proteinase cathepsin L expression correlates closely with the metastatic potential of H-ras-transformed murine fibroblasts. Oncogene. 1987;2(1):55–59. [PubMed] [Google Scholar]
- Fordham M. V., Burdge A. H., Matthews J., Williams G., Cooke T. Prostatic carcinoma cell DNA content measured by flow cytometry and its relation to clinical outcome. Br J Surg. 1986 May;73(5):400–403. doi: 10.1002/bjs.1800730530. [DOI] [PubMed] [Google Scholar]
- GRAY L. H., CONGER A. D., EBERT M., HORNSEY S., SCOTT O. C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953 Dec;26(312):638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- Garbisa S., Pozzatti R., Muschel R. J., Saffiotti U., Ballin M., Goldfarb R. H., Khoury G., Liotta L. A. Secretion of type IV collagenolytic protease and metastatic phenotype: induction by transfection with c-Ha-ras but not c-Ha-ras plus Ad2-E1a. Cancer Res. 1987 Mar 15;47(6):1523–1528. [PubMed] [Google Scholar]
- Gatenby R. A., Coia L. R., Richter M. P., Katz H., Moldofsky P. J., Engstrom P., Brown D. Q., Brookland R., Broder G. J. Oxygen tension in human tumors: in vivo mapping using CT-guided probes. Radiology. 1985 Jul;156(1):211–214. doi: 10.1148/radiology.156.1.4001408. [DOI] [PubMed] [Google Scholar]
- Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988 May;14(5):831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Groebe K., Vaupel P. Evaluation of oxygen diffusion distances in human breast cancer xenografts using tumor-specific in vivo data: role of various mechanisms in the development of tumor hypoxia. Int J Radiat Oncol Biol Phys. 1988 Sep;15(3):691–697. doi: 10.1016/0360-3016(88)90313-6. [DOI] [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Courtney A. H. Glucose consumption by transplanted tumors in vivo. Cancer Res. 1967 Jun;27(6):1031–1040. [PubMed] [Google Scholar]
- Hill R. P., Bush R. S. A new method of determining the fraction of hypoxic cells in a transplantable murine sarcoma. Radiat Res. 1977 Apr;70(1):141–153. [PubMed] [Google Scholar]
- Hill R. P., Stanley J. A. The response of hypoxic B16 melanoma cells to in vivo treatment with chemotherapeutic agents. Cancer Res. 1975 May;35(5):1147–1153. [PubMed] [Google Scholar]
- Horak E., Darling D. L., Tarin D. Analysis of organ-specific effects on metastatic tumor formation by studies in vitro. J Natl Cancer Inst. 1986 May;76(5):913–922. [PubMed] [Google Scholar]
- Jähde E., Glüsenkamp K. H., Rajewsky M. F. Increased drug cytotoxicity at reduced pH counteracts cyclophosphamide resistance in cultured rat mammary carcinoma cells. Int J Cancer. 1989 Dec 15;44(6):1082–1087. doi: 10.1002/ijc.2910440624. [DOI] [PubMed] [Google Scholar]
- Jähde E., Glüsenkamp K. H., Rajewsky M. F. Protection of cultured malignant cells from mitoxantrone cytotoxicity by low extracellular pH: a possible mechanism for chemoresistance in vivo. Eur J Cancer. 1990 Feb;26(2):101–106. doi: 10.1016/0277-5379(90)90290-a. [DOI] [PubMed] [Google Scholar]
- Kallinowski F., Vaupel P., Runkel S., Berg G., Fortmeyer H. P., Baessler K. H., Wagner K., Mueller-Klieser W., Walenta S. Glucose uptake, lactate release, ketone body turnover, metabolic micromilieu, and pH distributions in human breast cancer xenografts in nude rats. Cancer Res. 1988 Dec 15;48(24 Pt 1):7264–7272. [PubMed] [Google Scholar]
- Ling V., Chambers A. F., Harris J. F., Hill R. P. Quantitative genetic analysis of tumor progression. Cancer Metastasis Rev. 1985;4(2):173–192. doi: 10.1007/BF00050694. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Mueller-Klieser W., Walenta S., Paschen W., Kallinowski F., Vaupel P. Metabolic imaging in microregions of tumors and normal tissues with bioluminescence and photon counting. J Natl Cancer Inst. 1988 Aug 3;80(11):842–848. doi: 10.1093/jnci/80.11.842. [DOI] [PubMed] [Google Scholar]
- Newell K. J., Tannock I. F. Reduction of intracellular pH as a possible mechanism for killing cells in acidic regions of solid tumors: effects of carbonylcyanide-3-chlorophenylhydrazone. Cancer Res. 1989 Aug 15;49(16):4477–4482. [PubMed] [Google Scholar]
- Ossowski L., Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983 Dec;35(3 Pt 2):611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Rice G. C., Hoy C., Schimke R. T. Transient hypoxia enhances the frequency of dihydrofolate reductase gene amplification in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5978–5982. doi: 10.1073/pnas.83.16.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. C., Ling V., Schimke R. T. Frequencies of independent and simultaneous selection of Chinese hamster cells for methotrexate and doxorubicin (adriamycin) resistance. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9261–9264. doi: 10.1073/pnas.84.24.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R., Hughes C. S. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4843–4847. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R. The effects of glucose on protein synthesis and thermosensitivity in Chinese hamster ovary cells. J Biol Chem. 1983 Oct 25;258(20):12091–12093. [PubMed] [Google Scholar]
- Shen J., Hughes C., Chao C., Cai J., Bartels C., Gessner T., Subjeck J. Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3278–3282. doi: 10.1073/pnas.84.10.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V., Sadler J. G., Turner W. A., Kimpson J. J., Taylor J. D. Cathepsin B activity in B16 melanoma cells: a possible marker for metastatic potential. Cancer Res. 1982 Mar;42(3):980–986. [PubMed] [Google Scholar]
- Smith E., Stratford I. J., Adams G. E. Cytotoxicity of adriamycin on aerobic and hypoxic chinese hamster V79 cells in vitro. Br J Cancer. 1980 Oct;42(4):568–573. doi: 10.1038/bjc.1980.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subjeck J. R., Shyy T. T. Stress protein systems of mammalian cells. Am J Physiol. 1986 Jan;250(1 Pt 1):C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- Tannock I. F. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972 Jul;45(535):515–524. doi: 10.1259/0007-1285-45-535-515. [DOI] [PubMed] [Google Scholar]
- Tannock I. F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989 Aug 15;49(16):4373–4384. [PubMed] [Google Scholar]
- Tannock I. Response of aerobic and hypoxic cells in a solid tumor to adriamycin and cyclophosphamide and interaction of the drugs with radiation. Cancer Res. 1982 Dec;42(12):4921–4926. [PubMed] [Google Scholar]
- Tomasovic S. P., Welch D. R. Heat stress proteins and experimental cancer metastasis. Int J Hyperthermia. 1986 Jul-Sep;2(3):253–266. doi: 10.3109/02656738609016484. [DOI] [PubMed] [Google Scholar]
- Turner G. A. Increased release of tumour cells by collagenase at acid pH: a possible mechanism for metastasis. Experientia. 1979 Dec 15;35(12):1657–1658. doi: 10.1007/BF01953252. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Welch W. J. The mammalian heat shock (or stress) response: a cellular defense mechanism. Adv Exp Med Biol. 1987;225:287–304. doi: 10.1007/978-1-4684-5442-0_26. [DOI] [PubMed] [Google Scholar]
- Whelan S. A., Hightower L. E. Differential induction of glucose-regulated and heat shock proteins: effects of pH and sulfhydryl-reducing agents on chicken embryo cells. J Cell Physiol. 1985 Nov;125(2):251–258. doi: 10.1002/jcp.1041250212. [DOI] [PubMed] [Google Scholar]
- Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984 Dec;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- Yamori T., Iida H., Tsukagoshi S., Tsuruo T. Growth stimulating activity of lung extract on lung-colonizing colon 26 clones and its partial characterization. Clin Exp Metastasis. 1988 Mar-Apr;6(2):131–139. doi: 10.1007/BF01784844. [DOI] [PubMed] [Google Scholar]
- Young S. D., Hill R. P. Dynamic heterogeneity: isolation of murine tumor cell populations enriched for metastatic variants and quantification of the unstable expression of the phenotype. Clin Exp Metastasis. 1986 Jul-Sep;4(3):153–176. doi: 10.1007/BF00117930. [DOI] [PubMed] [Google Scholar]
- Young S. D., Hill R. P. Effects of reoxygenation on cells from hypoxic regions of solid tumors: analysis of transplanted murine tumors for evidence of DNA overreplication. Cancer Res. 1990 Aug 15;50(16):5031–5038. [PubMed] [Google Scholar]
- Young S. D., Hill R. P. Effects of reoxygenation on cells from hypoxic regions of solid tumors: anticancer drug sensitivity and metastatic potential. J Natl Cancer Inst. 1990 Mar 7;82(5):371–380. doi: 10.1093/jnci/82.5.371. [DOI] [PubMed] [Google Scholar]
- Young S. D., Marshall R. S., Hill R. P. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9533–9537. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman P. V., Hawson G. A., Bint M. H., Parsons P. G. Ploidy as a prognostic determinant in surgically treated lung cancer. Lancet. 1987 Sep 5;2(8558):530–533. doi: 10.1016/s0140-6736(87)92923-0. [DOI] [PubMed] [Google Scholar]


