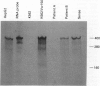
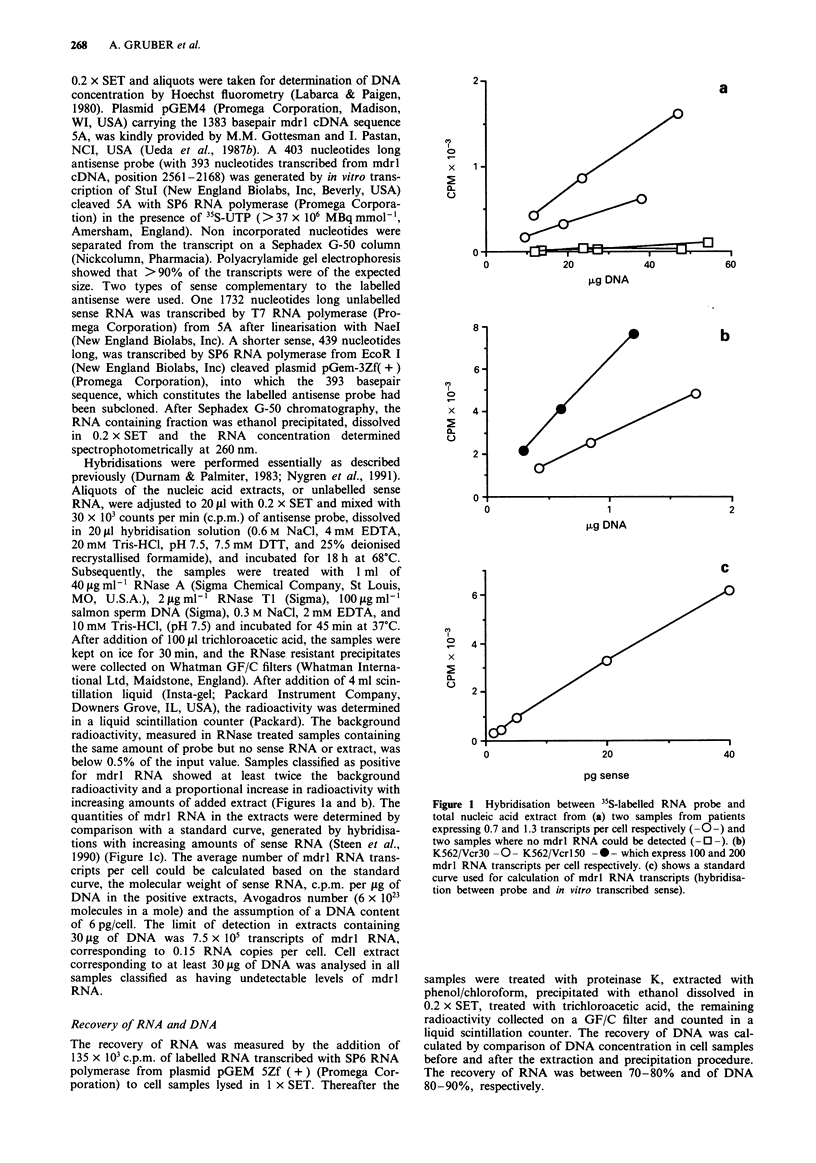
Abstract
By using a quantitative RNA-RNA solution hybridisation method, the average number of mdr1 RNA transcripts per cell was measured in total nucleic acid extracts of leukaemic cells from patients with acute leukaemia. The results in different types of leukaemia were (number of patients with detectable mdr1 RNA/total number of patients; median number of transcripts per cell in samples with detectable mdr1 RNA); de novo untreated acute myelocytic leukaemia (AML): 20/44; 0.7, secondary acute myelocytic leukaemia: 8/13; 1.1, acute lymphocytic (ALL) and undifferentiated leukaemia: 5/14; 0.6, relapsed leukaemia: 7/15; 0.7. Forty-six patients with de novo untreated acute leukaemia (AML: n = 34, ALL: n = 12) were evaluable for response to induction chemotherapy. Twelve of 18 patients (67%) with detectable mdr1 RNA levels achieved complete remission compared to 23 of 28 (82%) with undetectable levels (P = 0.40). The remission duration tended to be longer among patients with undetectable mdr1 RNA (P = 0.08). Leukaemic cells were analysed on consecutive occasions in 12 patients. The level of expression increased in four and decreased in two. In conclusion, expression of mdr1 RNA is common in acute untreated leukaemia. However, treatment with cytostatic drugs seems only rarely to increase the proportion of leukaemic cells that express mdr1 RNA. Expression of the mdr1 gene could be one of several equally important factors contributing to drug resistance in acute leukaemia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. T. Unknotting the complexities of multidrug resistance: the involvement of DNA topoisomerases in drug action and resistance. J Natl Cancer Inst. 1989 Nov 15;81(22):1683–1685. doi: 10.1093/jnci/81.22.1683. [DOI] [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Haddad G., Thorner P. S., DeBoer G., Lin Y. P., Ondrusek N., Yeger H., Ling V. P-glycoprotein expression as a predictor of the outcome of therapy for neuroblastoma. N Engl J Med. 1991 Dec 5;325(23):1608–1614. doi: 10.1056/NEJM199112053252304. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Thorner P. S., Haddad G., Ling V. Immunohistochemical detection of P-glycoprotein: prognostic correlation in soft tissue sarcoma of childhood. J Clin Oncol. 1990 Apr;8(4):689–704. doi: 10.1200/JCO.1990.8.4.689. [DOI] [PubMed] [Google Scholar]
- Chin J. E., Soffir R., Noonan K. E., Choi K., Roninson I. B. Structure and expression of the human MDR (P-glycoprotein) gene family. Mol Cell Biol. 1989 Sep;9(9):3808–3820. doi: 10.1128/mcb.9.9.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks M. K., Yalowich J. C., Beck W. T. Atypical multiple drug resistance in a human leukemic cell line selected for resistance to teniposide (VM-26). Cancer Res. 1987 Mar 1;47(5):1297–1301. [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Favrot M., Combaret V., Goillot E., Wagner J. P., Bouffet E., Mazingue F., Thyss A., Bordigoni P., Delsol G., Bailly C. Expression of P-glycoprotein restricted to normal cells in neuroblastoma biopsies. Br J Cancer. 1991 Aug;64(2):233–238. doi: 10.1038/bjc.1991.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Roninson I., Varshavsky A., Housman D. E. Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):337–341. doi: 10.1073/pnas.83.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker W. G., Slade D. L., Dalton W. S., Meltzer P. S., Trent J. M. Multidrug resistance in mitoxantrone-selected HL-60 leukemia cells in the absence of P-glycoprotein overexpression. Cancer Res. 1989 Aug 15;49(16):4542–4549. [PubMed] [Google Scholar]
- Herweijer H., Sonneveld P., Baas F., Nooter K. Expression of mdr1 and mdr3 multidrug-resistance genes in human acute and chronic leukemias and association with stimulation of drug accumulation by cyclosporine. J Natl Cancer Inst. 1990 Jul 4;82(13):1133–1140. doi: 10.1093/jnci/82.13.1133. [DOI] [PubMed] [Google Scholar]
- Holmes J., Wareing C., Jacobs A., Hayes J. D., Padua R. A., Wolf C. R. Glutathione-s-transferase pi expression in leukaemia: a comparative analysis with mdr-1 data. Br J Cancer. 1990 Aug;62(2):209–212. doi: 10.1038/bjc.1990.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tanimoto M., Kumazawa T., Okumura M., Morishima Y., Ohno R., Saito H. Increased P-glycoprotein expression and multidrug-resistant gene (mdr1) amplification are infrequently found in fresh acute leukemia cells. Sequential analysis of 15 cases at initial presentation and relapsed stage. Cancer. 1989 Apr 15;63(8):1534–1538. doi: 10.1002/1097-0142(19890415)63:8<1534::aid-cncr2820630813>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Kuwazuru Y., Yoshimura A., Hanada S., Utsunomiya A., Makino T., Ishibashi K., Kodama M., Iwahashi M., Arima T., Akiyama S. Expression of the multidrug transporter, P-glycoprotein, in acute leukemia cells and correlation to clinical drug resistance. Cancer. 1990 Sep 1;66(5):868–873. doi: 10.1002/1097-0142(19900901)66:5<868::aid-cncr2820660510>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Marie J. P., Zittoun R., Sikic B. I. Multidrug resistance (mdr1) gene expression in adult acute leukemias: correlations with treatment outcome and in vitro drug sensitivity. Blood. 1991 Aug 1;78(3):586–592. [PubMed] [Google Scholar]
- Musto P., Melillo L., Lombardi G., Matera R., di Giorgio G., Carotenuto M. High risk of early resistant relapse for leukaemic patients with presence of multidrug resistance associated P-glycoprotein positive cells in complete remission. Br J Haematol. 1991 Jan;77(1):50–53. doi: 10.1111/j.1365-2141.1991.tb07947.x. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren P., Larsson R., Gruber A., Peterson C., Bergh J. Doxorubicin selected multidrug-resistant small cell lung cancer cell lines characterised by elevated cytoplasmic Ca2+ and resistance modulation by verapamil in absence of P-glycoprotein overexpression. Br J Cancer. 1991 Dec;64(6):1011–1018. doi: 10.1038/bjc.1991.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker R., Wallner J., Geissler K., Linkesch W., Haas O. A., Bettelheim P., Hopfner M., Scherrer R., Valent P., Havelec L. MDR1 gene expression and treatment outcome in acute myeloid leukemia. J Natl Cancer Inst. 1991 May 15;83(10):708–712. doi: 10.1093/jnci/83.10.708. [DOI] [PubMed] [Google Scholar]
- Sato H., Preisler H., Day R., Raza A., Larson R., Browman G., Goldberg J., Vogler R., Grunwald H., Gottlieb A. MDR1 transcript levels as an indication of resistant disease in acute myelogenous leukaemia. Br J Haematol. 1990 Jul;75(3):340–345. doi: 10.1111/j.1365-2141.1990.tb04346.x. [DOI] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., de Lange J. H., Pinedo H. M., van Heijningen T. H., Kuiper C. M., Scheffer G. L., Scheper R. J., van Kalken C. K., Baak J. P. Early multidrug resistance, defined by changes in intracellular doxorubicin distribution, independent of P-glycoprotein. Br J Cancer. 1991 Nov;64(5):857–861. doi: 10.1038/bjc.1991.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld P., Nooter K. Reversal of drug-resistance by cyclosporin-A in a patient with acute myelocytic leukaemia. Br J Haematol. 1990 Jun;75(2):208–211. doi: 10.1111/j.1365-2141.1990.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Steen A. M., Luthman H., Hellgren D., Lambert B. Levels of hypoxanthine phosphoribosyltransferase RNA in human cells. Exp Cell Res. 1990 Feb;186(2):236–244. doi: 10.1016/0014-4827(90)90301-p. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Dalton W. S., Parrish P. R., Gleason M. C., Bellamy W. T., Thompson F. H., Roe D. J., Trent J. M. Different mechanisms of decreased drug accumulation in doxorubicin and mitoxantrone resistant variants of the MCF7 human breast cancer cell line. Br J Cancer. 1991 Jun;63(6):923–929. doi: 10.1038/bjc.1991.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Nojiri M., Tsukagoshi S., Sakurai Y. Circumvention of vincristine and Adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res. 1983 Jun;43(6):2905–2910. [PubMed] [Google Scholar]
- Twentyman P. R., Reeve J. G., Koch G., Wright K. A. Chemosensitisation by verapamil and cyclosporin A in mouse tumour cells expressing different levels of P-glycoprotein and CP22 (sorcin). Br J Cancer. 1990 Jul;62(1):89–95. doi: 10.1038/bjc.1990.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Clark D. P., Chen C. J., Roninson I. B., Gottesman M. M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem. 1987 Jan 15;262(2):505–508. [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Ten Houte de Lange T., Kooiman P. M., Van der Velde-Koerts T., Borst P. The human mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. EMBO J. 1987 Nov;6(11):3325–3331. doi: 10.1002/j.1460-2075.1987.tb02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Van der Velde-Koerts T., Biedler J. L., Meyers M. B., Ozols R. F., Hamilton T. C., Joenje H., Borst P. Genes amplified and overexpressed in human multidrug-resistant cell lines. Cancer Res. 1988 Nov 1;48(21):5927–5932. [PubMed] [Google Scholar]