Abstract
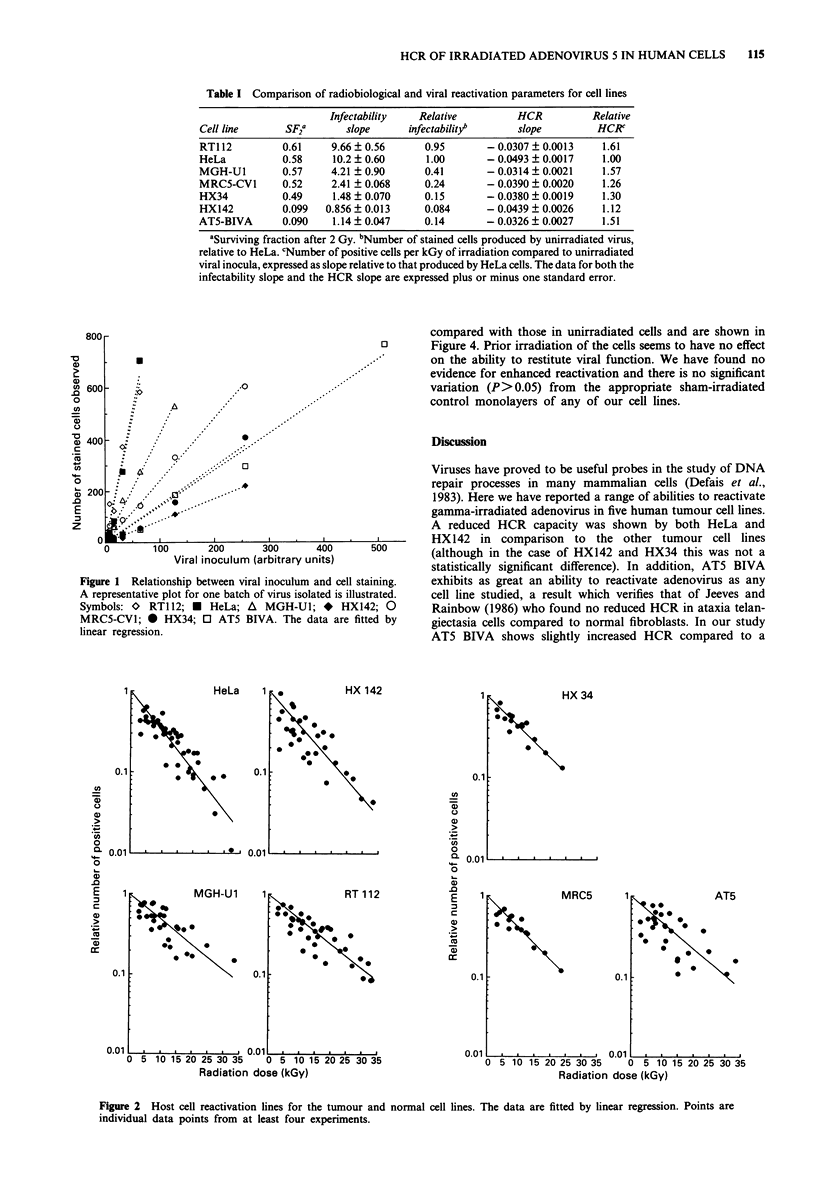
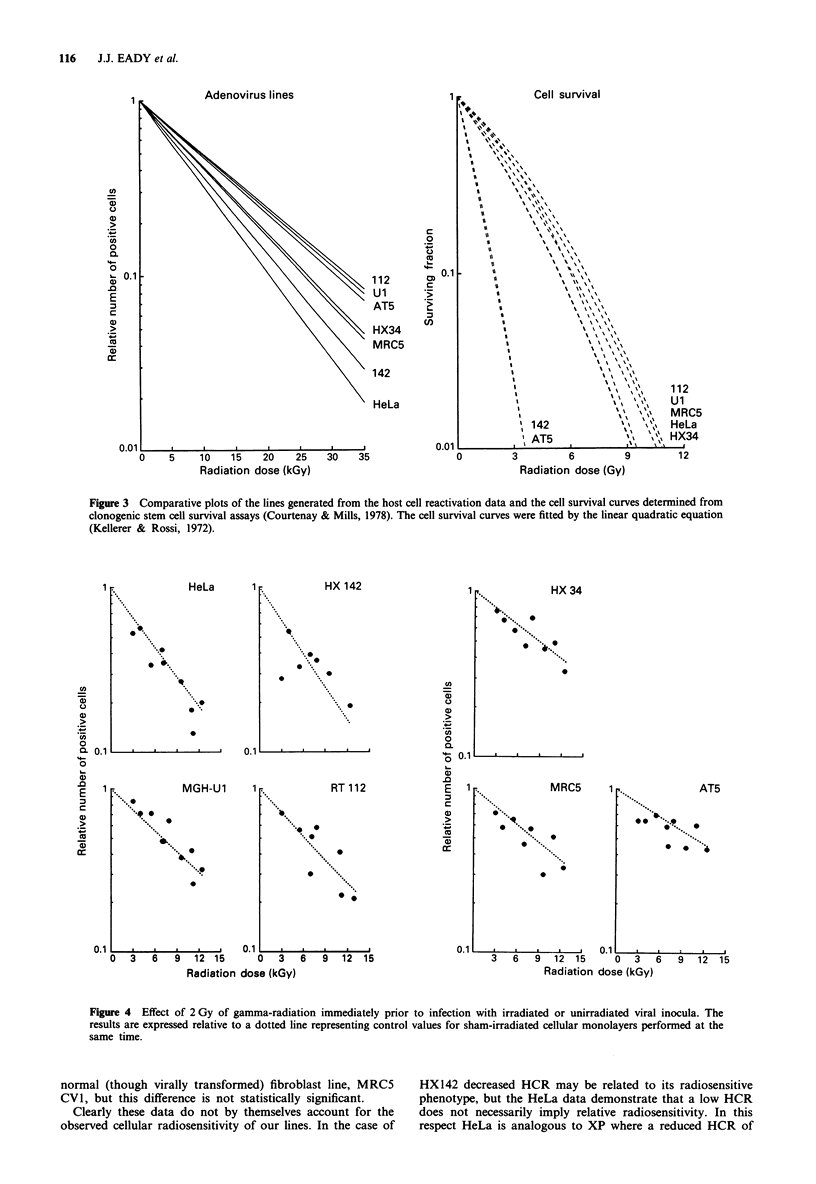
DNA repair processes play an important role in the determination of radiation response in both normal and tumour cells. We have investigated one aspect of DNA repair in a number of human cell lines of varying radiosensitivity using the adenovirus 5 host cell reactivation assay (HCR). In this technique, gamma-irradiated virions are used to infect cells and the ability of the cellular repair systems to process this damage is assayed by a convenient immunoperoxidase method recognising viral structural antigen expression on the cell membrane 48 h after infection. Reduced HCR was exhibited by radioresistant HeLa cells and by a radiosensitive neuroblastoma cell line, HX142. In contrast, an ataxia telangiectasia cell line, AT5 BIVA, did not show reduced HCR. On the basis of these results we can make no general conclusions about the relevance of HCR to cellular radiosensitivity. We have extended these studies to determine whether our cell lines exhibited enhanced viral reactivation (ER) following a small priming dose of gamma-radiation given to the cells before viral infection. No evidence for this phenomenon was found either in normal or tumour cell lines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlett C. F., Green M. H., Priestley A., Harcourt S. A., Mayne L. V. Comparative human cellular radiosensitivity: I. The effect of SV40 transformation and immortalisation on the gamma-irradiation survival of skin derived fibroblasts from normal individuals and from ataxia-telangiectasia patients and heterozygotes. Int J Radiat Biol. 1988 Dec;54(6):911–928. doi: 10.1080/09553008814552321. [DOI] [PubMed] [Google Scholar]
- Coquerelle T. M., Weibezahn K. F. Rejoining of DNA double-strand breaks in human fibroblasts and its impairment in one ataxia telangiectasia and two Fanconi strains. J Supramol Struct Cell Biochem. 1981;17(4):369–376. doi: 10.1002/jsscb.380170408. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Debenham P. G., Masson W. K., Webb M. B. Ataxia-telangiectasia: a human mutation giving high-frequency misrepair of DNA double-stranded scissions. Mol Biol Med. 1986 Jun;3(3):229–244. [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H. Human brain tumour cell strains with deficient host-cell reactivation of N-methyl-N'-nitro-N-nitrosoguanidine-damaged adenovirus 5. Nature. 1979 Jun 28;279(5716):797–799. doi: 10.1038/279797a0. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Deacon J. M., Wilson P. A., Peckham M. J. The radiobiology of human neuroblastoma. Radiother Oncol. 1985 Apr;3(3):201–209. doi: 10.1016/s0167-8140(85)80029-3. [DOI] [PubMed] [Google Scholar]
- Hayward I. P., Parsons P. G. Comparison of virus reactivation, DNA base damage, and cell cycle effects in autologous human melanoma cells resistant to methylating agents. Cancer Res. 1984 Jan;44(1):55–58. [PubMed] [Google Scholar]
- Hilgers G., Abrahams P. J., Chen Y. Q., Schouten R., Cornelis J. J., Lowe J. E., van der Eb A. J., Rommelaere J. Impaired recovery and mutagenic SOS-like responses in ataxia telangiectasia cells. Mutagenesis. 1989 Jul;4(4):271–276. doi: 10.1093/mutage/4.4.271. [DOI] [PubMed] [Google Scholar]
- Hilgers G., Chen Y. Q., Cornelis J. J., Rommelaere J. Deficient expression of enhanced reactivation of parvovirus H-1 in ataxia telangiectasia cells irradiated with X-rays or u.v. light. Carcinogenesis. 1987 Feb;8(2):315–319. doi: 10.1093/carcin/8.2.315. [DOI] [PubMed] [Google Scholar]
- Jeeves W. P., Rainbow A. J. An aberration in gamma-ray-enhanced reactivation of irradiated adenovirus in ataxia telangiectasia fibroblasts. Carcinogenesis. 1986 Mar;7(3):381–387. doi: 10.1093/carcin/7.3.381. [DOI] [PubMed] [Google Scholar]
- Jeeves W. P., Rainbow A. J. Gamma-ray enhanced reactivation of gamma-irradiated adenovirus in human cells. Biochem Biophys Res Commun. 1979 Sep 27;90(2):567–574. doi: 10.1016/0006-291x(79)91273-7. [DOI] [PubMed] [Google Scholar]
- Jeeves W. P., Rainbow A. J. U.V. enhanced reactivation of U.V.-and gamma-irradiated adenovirus in Cockayne syndrome and Xeroderma pigmentosum fibroblasts. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Jun;43(6):625–647. doi: 10.1080/09553008314550731. [DOI] [PubMed] [Google Scholar]
- Jeeves W. P., Rainbow A. J. U.V. enhanced reactivation of U.V.-and gamma-irradiated adenovirus in normal human fibroblasts. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Jun;43(6):599–623. doi: 10.1080/09553008314550721. [DOI] [PubMed] [Google Scholar]
- Kato T., Irwin R. J., Jr, Prout G. R., Jr Cell cycles in two cell lines of human bladder carcinoma. Tohoku J Exp Med. 1977 Feb;121(2):157–164. doi: 10.1620/tjem.121.157. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Sedgwick S. G., Jeggo P. A. X-ray sensitive mutants of Chinese hamster ovary cells defective in double-strand break rejoining. Mutat Res. 1984 Nov-Dec;132(5-6):189–196. doi: 10.1016/0167-8817(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Masters J. R., Hepburn P. J., Walker L., Highman W. J., Trejdosiewicz L. K., Povey S., Parkar M., Hill B. T., Riddle P. R., Franks L. M. Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines. Cancer Res. 1986 Jul;46(7):3630–3636. [PubMed] [Google Scholar]
- McMillan T. J., Cassoni A. M., Edwards S., Holmes A., Peacock J. H. The relationship of DNA double-strand break induction to radiosensitivity in human tumour cell lines. Int J Radiat Biol. 1990 Sep;58(3):427–438. doi: 10.1080/09553009014551781. [DOI] [PubMed] [Google Scholar]
- Parsons P. G., Maynard K. R., Little J. H., McLeod G. R. Adenovirus replication as an in vitro probe for drug sensitivity in human tumors. Eur J Cancer Clin Oncol. 1986 Apr;22(4):401–409. doi: 10.1016/0277-5379(86)90105-7. [DOI] [PubMed] [Google Scholar]
- Peacock J. H., Cassoni A. M., McMillan T. J., Steel G. G. Radiosensitive human tumour cell lines may not be recovery deficient. Int J Radiat Biol. 1988 Dec;54(6):945–953. doi: 10.1080/09553008814552341. [DOI] [PubMed] [Google Scholar]
- Peacock J. H., Eady J. J., Edwards S., Holmes A., McMillan T. J., Steel G. G. Initial damage or repair as the major determinant of cellular radiosensitivity? Int J Radiat Biol. 1989 Nov;56(5):543–547. doi: 10.1080/09553008914551711. [DOI] [PubMed] [Google Scholar]
- Radford I. R. Evidence for a general relationship between the induced level of DNA double-strand breakage and cell-killing after X-irradiation of mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Apr;49(4):611–620. doi: 10.1080/09553008514552861. [DOI] [PubMed] [Google Scholar]
- Rainbow A. J. Defective repair of UV-damaged DNA in human tumor and SV40-transformed human cells but not in adenovirus-transformed human cells. Carcinogenesis. 1989 Jun;10(6):1073–1077. doi: 10.1093/carcin/10.6.1073. [DOI] [PubMed] [Google Scholar]
- Rainbow A. J. Host cell reactivation of sunlamp-exposed adenovirus in fibroblasts from patients with Bloom's syndrome, ataxia telangiectasia, and Huntington's disease. Environ Mol Mutagen. 1991;17(2):98–103. doi: 10.1002/em.2850170206. [DOI] [PubMed] [Google Scholar]
- Rainbow A. J., Howes M. Decreased repair of gamma-irradiated adenovirus in Xeroderma pigmentosum fibroblasts. Int J Radiat Biol Relat Stud Phys Chem Med. 1979 Dec;36(6):621–629. doi: 10.1080/09553007914551451. [DOI] [PubMed] [Google Scholar]
- Rainbow A. J., Mak S. DNA damage and biological function of human adenovirus after u.v.-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Jul;24(1):59–72. doi: 10.1080/09553007314550821. [DOI] [PubMed] [Google Scholar]
- Rainbow A. J., Mak S. DNA strand breakage and biological functions of human adenovirus after gamma irradiation. Radiat Res. 1972 May;50(2):319–333. [PubMed] [Google Scholar]
- Rainbow A. J. Reduced capacity to repair irradiated adenovirus in fibroblasts from xeroderma pigmentosum heterozygotes. Cancer Res. 1980 Nov;40(11):3945–3949. [PubMed] [Google Scholar]
- Rainbow A. J. Repair of radiation-induced DNA breaks in human adenovirus. Radiat Res. 1974 Oct;60(1):155–164. [PubMed] [Google Scholar]
- Rösen A., Taylor D. M., Darai G. Influencing of ionizing radiation on herpes simplex virus and its genome. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Nov;52(5):795–804. doi: 10.1080/09553008714552311. [DOI] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Carcinogens enhance survival of UV-irradiated simian virus 40 in treated monkey kidney cells: induction of a recovery pathway? Proc Natl Acad Sci U S A. 1978 Jan;75(1):346–350. doi: 10.1073/pnas.75.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadley J. D., Wiencke J. K. Induction of the adaptive response by X-rays is dependent on radiation intensity. Int J Radiat Biol. 1989 Jul;56(1):107–118. doi: 10.1080/09553008914551231. [DOI] [PubMed] [Google Scholar]
- Smith I. E., Courtenay V. D., Mills J., Peckham M. J. In vitro radiation response of cells from four human tumors propagated in immune-suppressed mice. Cancer Res. 1978 Feb;38(2):390–392. [PubMed] [Google Scholar]
- Taylor A. M. Unrepaired DNA strand breaks in irradiated ataxia telangiectasia lymphocytes suggested from cytogenetic observations. Mutat Res. 1978 Jun;50(3):407–418. doi: 10.1016/0027-5107(78)90045-3. [DOI] [PubMed] [Google Scholar]


