Abstract
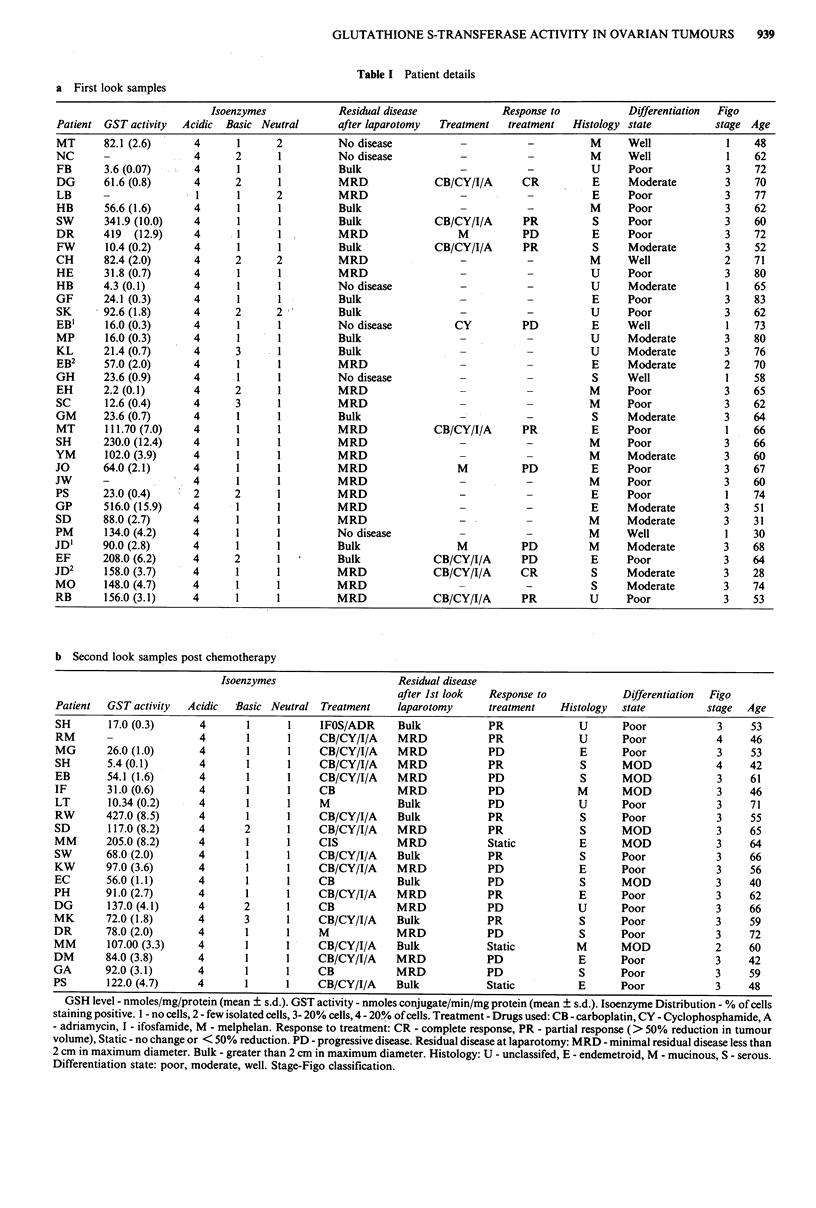
A study involving the measurement of glutathione S-transferase activities and isoenzyme distributions in human ovarian tumours has been carried out. These tumours have been obtained either at initial debulking surgery, prior to cytotoxic chemotherapy, or at second look laparotomy following chemotherapy. The response rates of these two groups to chemotherapy differ markedly, with patients who have relapsed following initial chemotherapy showing a reduction in response rates to subsequent chemotherapy. Analysis of these data show no statistically significant differences between the glutathione S-transferase activity or isoenzyme distribution in these two groups of patients. Significant differences were observed in the glutathione-S-transferase activities (GST) between tumours and normal ovaries. GST activities in pre-chemotherapy tumours (n = 33, P = 0.01) and post-chemotherapy tumours (n = 20, P = 0.001) where significantly higher than the GST activity in normal ovaries (n = 15). One feature was the expression of the basic isoenzyme which is expressed more in normal ovaries than in tumours. No differences in these parameters were observed in normal peritoneal tissue taken from patients before or after chemotherapy. These data do not support the hypothesis that changes in glutathione S-transferase enzyme activity or isoenzyme expression are major determinants of response to chemotherapy in ovarian tumours.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujiwara Y., Sugimoto Y., Kasahara K., Bungo M., Yamakido M., Tew K. D., Saijo N. Determinants of drug response in a cisplatin-resistant human lung cancer cell line. Jpn J Cancer Res. 1990 May;81(5):527–535. doi: 10.1111/j.1349-7006.1990.tb02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney H., Crowther D., Anderson H., Murphy D., Prendiville J., Ranson M., Mayor P., Swindell R., Buckley C. H., Tindall V. R. Five year follow-up and dose delivery analysis of cisplatin, iproplatin or carboplatin in combination with cyclophosphamide in advanced ovarian carcinoma. Ann Oncol. 1990 Nov;1(6):427–433. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hall A., Foster S., Proctor S. J., Cattan A. R. Purification and characterization of a pi class glutathione S-transferase from human leukaemic cells. Br J Haematol. 1990 Dec;76(4):494–500. doi: 10.1111/j.1365-2141.1990.tb07906.x. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hayes J. D., Wolf C. R. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis. 1988 Jul;9(7):1283–1287. doi: 10.1093/carcin/9.7.1283. [DOI] [PubMed] [Google Scholar]
- McGown A. T., Fox B. W. A proposed mechanism of resistance to cyclophosphamide and phosphoramide mustard in a Yoshida cell line in vitro. Cancer Chemother Pharmacol. 1986;17(3):223–226. doi: 10.1007/BF00256688. [DOI] [PubMed] [Google Scholar]
- Mistry P., Kelland L. R., Abel G., Sidhar S., Harrap K. R. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian carcinoma cell lines. Br J Cancer. 1991 Aug;64(2):215–220. doi: 10.1038/bjc.1991.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K., Saijo N., Tsuchida S., Sakai M., Tsunokawa Y., Yokota J., Muramatsu M., Sato K., Terada M., Tew K. D. Glutathione-S-transferase pi as a determinant of drug resistance in transfectant cell lines. J Biol Chem. 1990 Mar 15;265(8):4296–4301. [PubMed] [Google Scholar]
- Nash J. D., Young R. C. Gynecologic malignancies. Cancer Chemother Biol Response Modif. 1988;10:291–312. [PubMed] [Google Scholar]
- Ozols R. F., Young R. C. Chemotherapy of ovarian cancer. Semin Oncol. 1984 Sep;11(3):251–263. [PubMed] [Google Scholar]
- Randall B. J., Angus B., Akiba R., Hall A., Cattan A. R., Proctor S. J., Jones R. A., Horne C. H. Glutathione S-transferase (placental) as a marker of transformation in the human cervix uteri: an immunohistochemical study. Br J Cancer. 1990 Oct;62(4):614–618. doi: 10.1038/bjc.1990.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwelling L. A. Cisplatin and new platinum analogs. Cancer Chemother Biol Response Modif. 1988;10:64–72. [PubMed] [Google Scholar]



