Abstract
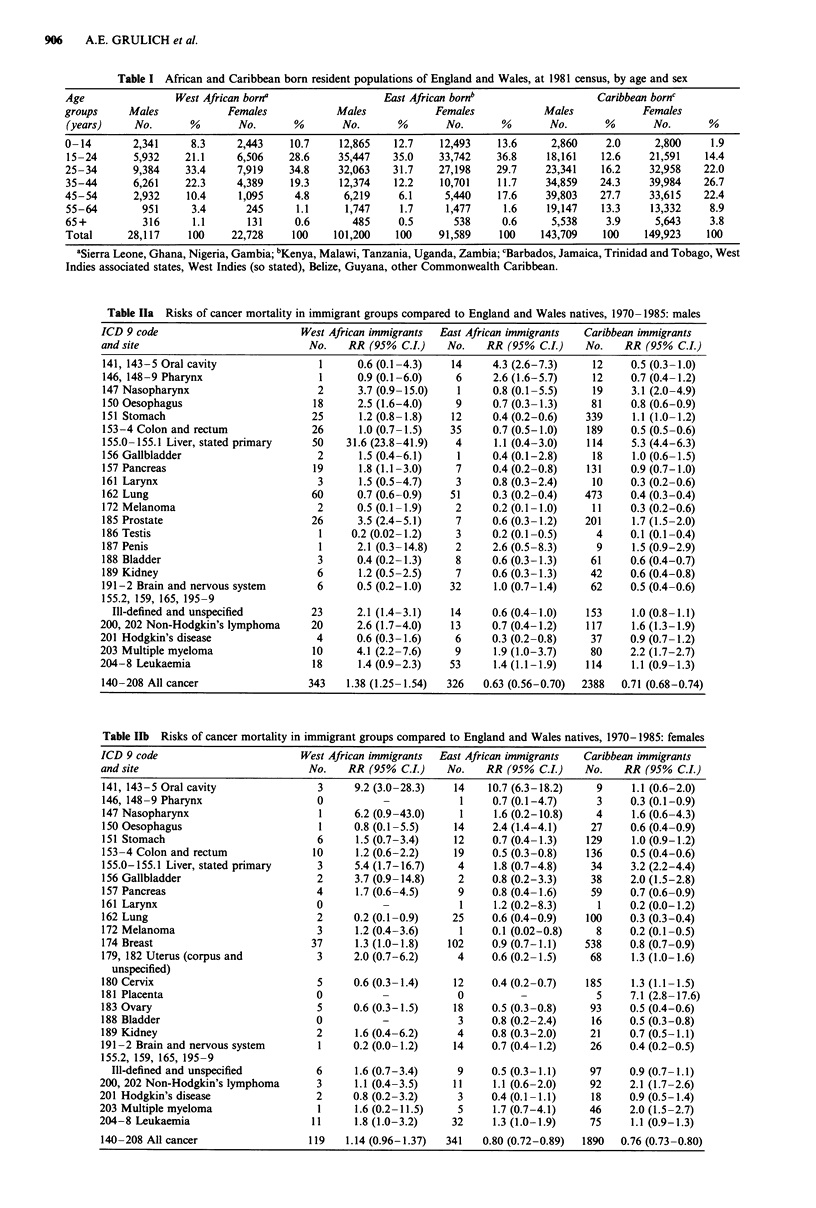
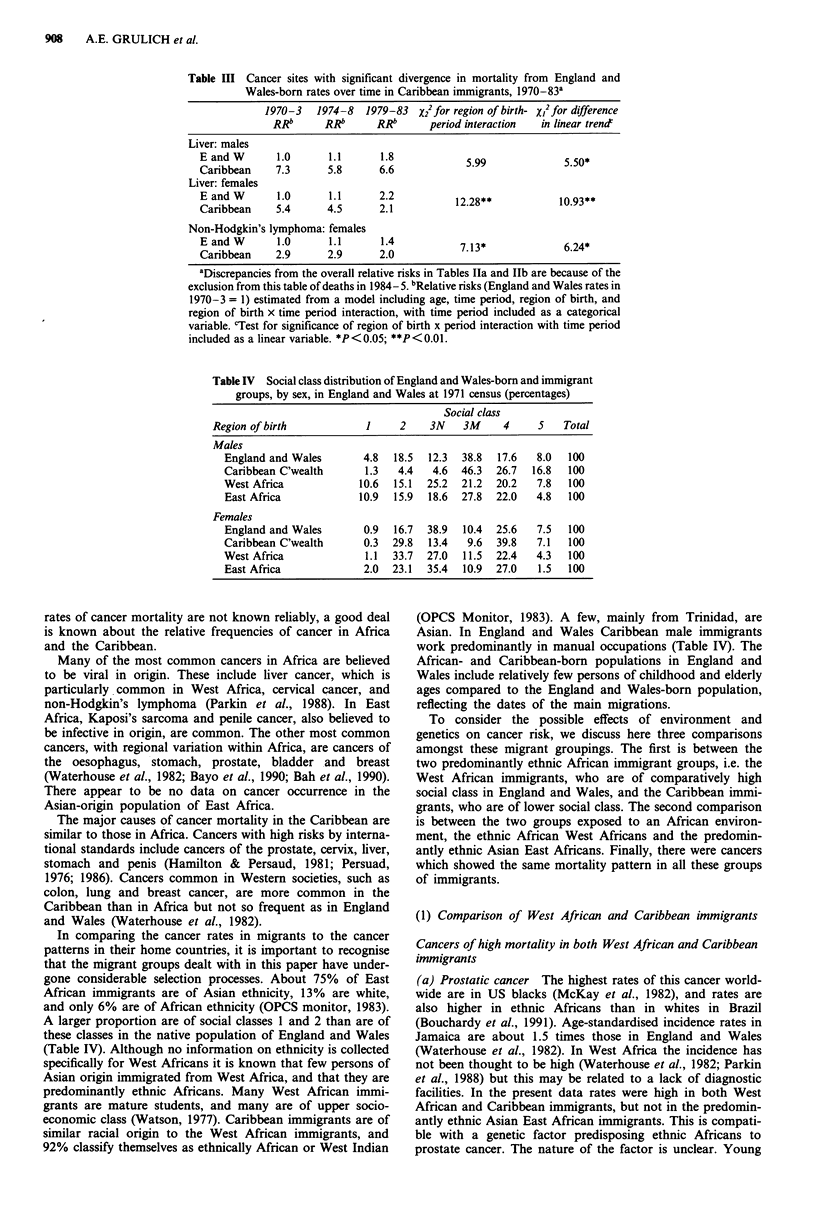
Cancer mortality during 1970-85 of immigrants from East and West Africa and the Caribbean to England and Wales is described. Overall cancer mortality was raised in West African males (RR 1.38, 95% CI 1.25-1.54), and non-significantly raised in West African females (RR 1.14, 0.96-1.37) compared to mortality in the England and Wales-born population. Much of the increased risk was due to very high rates of liver cancer in males (RR 31.6, 23.8-41.9), but rates were also raised for a wide range of other cancers in each sex. Only lung and brain cancer had significantly decreased mortality. In East Africans, overall cancer mortality was low in males (RR 0.63, 0.56-0.70), and in females (RR 0.80, 0.72-0.89). Mortality was significantly low for cancers of the stomach, pancreas and testis, and Hodgkin's disease in males, for cervical cancer in females, and for lung cancer and melanoma in both sexes. Cancer sites with significantly raised mortality included oropharyngeal cancer, leukaemia, and multiple myeloma in both sexes. In Caribbean immigrants overall cancer rates were significantly low in males (RR 0.71, 0.68-0.74) and in females (RR 0.76, 0.73-0.80). Mortality was significantly low for many cancers including colorectal, lung, testis and brain cancers. Mortality was significantly raised only for cancer of the prostate in males, of the placenta in females, and of the liver, non-Hodgkin's lymphoma and multiple myeloma in both sexes. Overall, mortality was high from prostatic cancer and liver cancer, and was low from brain cancer, in predominantly ethnic African immigrant groups. Both East and West African immigrants had raised rates of leukaemia. All of the migrant groups had high rates of multiple myeloma and low rates of testicular, ovarian and lung cancer. Genetic and environmental factors that may contribute to these patterns are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bah E., Hall A. J., Inskip H. M. The first 2 years of the Gambian National Cancer Registry. Br J Cancer. 1990 Oct;62(4):647–650. doi: 10.1038/bjc.1990.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayo S., Parkin D. M., Koumaré A. K., Diallo A. N., Ba T., Soumaré S., Sangaré S. Cancer in Mali, 1987-1988. Int J Cancer. 1990 Apr 15;45(4):679–684. doi: 10.1002/ijc.2910450418. [DOI] [PubMed] [Google Scholar]
- Bouchardy C., Mirra A. P., Khlat M., Parkin D. M., de Souza J. M., Gotlieb S. L. Ethnicity and cancer risk in São Paulo, Brazil. Cancer Epidemiol Biomarkers Prev. 1991 Nov-Dec;1(1):21–27. [PubMed] [Google Scholar]
- Brownson R. C., Reif J. S., Chang J. C., Davis J. R. An analysis of occupational risks for brain cancer. Am J Public Health. 1990 Feb;80(2):169–172. doi: 10.2105/ajph.80.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson B. Epidemiological aspects of the transmission of hepatitis B by HBsAg-positive adopted children. Scand J Infect Dis. 1986;18(2):105–109. doi: 10.3109/00365548609032315. [DOI] [PubMed] [Google Scholar]
- Christopherson W. M., Nealon N. A. Uterine cancer: a comparative study of black and white women. Prog Clin Biol Res. 1981;53:185–195. [PubMed] [Google Scholar]
- Coursaget P., Chiron J. P., Barres J. L., Barin F., Cottey P., Tortey E., Yvonnet B., Diop B., MBoup S., Diop-Mar I. Hepatitis B virus serological markers in Africans with liver cirrhosis and hepatocellular carcinoma. IARC Sci Publ. 1984;(63):181–198. [PubMed] [Google Scholar]
- Crombie I. K. Racial differences in melanoma incidence. Br J Cancer. 1979 Aug;40(2):185–193. doi: 10.1038/bjc.1979.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton P. J., Persaud V. Cancer among blacks in the West Indies. Prog Clin Biol Res. 1981;53:1–15. [PubMed] [Google Scholar]
- Jackson M. A., Kovi J., Heshmat M. Y., Jones G. W., Rao M. S., Ahluwalia B. S. Factors involved in the high incidence of prostatic cancer among American blacks. Prog Clin Biol Res. 1981;53:111–132. [PubMed] [Google Scholar]
- Kaldor J., Khlat M., Parkin D. M., Shiboski S., Steinitz R. Log-linear models for cancer risk among migrants. Int J Epidemiol. 1990 Jun;19(2):233–239. doi: 10.1093/ije/19.2.233. [DOI] [PubMed] [Google Scholar]
- Parkin D. M., Lärä E., Muir C. S. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer. 1988 Feb 15;41(2):184–197. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- Persaud V. Cancer control in the Commonwealth Caribbean. Recommendations for a regional approach. West Indian Med J. 1986 Sep;35(3):145–148. [PubMed] [Google Scholar]
- Persaud V. Cancer incidence in Jamaica. An 18-year analysis (1958--1975). West Indian Med J. 1976 Dec;25(4):210–215. [PubMed] [Google Scholar]
- Ross R., Bernstein L., Judd H., Hanisch R., Pike M., Henderson B. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst. 1986 Jan;76(1):45–48. [PubMed] [Google Scholar]
- Smith G. D., Leon D., Shipley M. J., Rose G. Socioeconomic differentials in cancer among men. Int J Epidemiol. 1991 Jun;20(2):339–345. doi: 10.1093/ije/20.2.339. [DOI] [PubMed] [Google Scholar]
- Vall Mayans M., Hall A. J., Inskip H. M., Chotard J., Lindsay S. W., Coromina E., Mendy M., Alonso P. L., Whittle H. Risk factors for transmission of hepatitis B virus to Gambian children. Lancet. 1990 Nov 3;336(8723):1107–1109. doi: 10.1016/0140-6736(90)92580-b. [DOI] [PubMed] [Google Scholar]


