Abstract
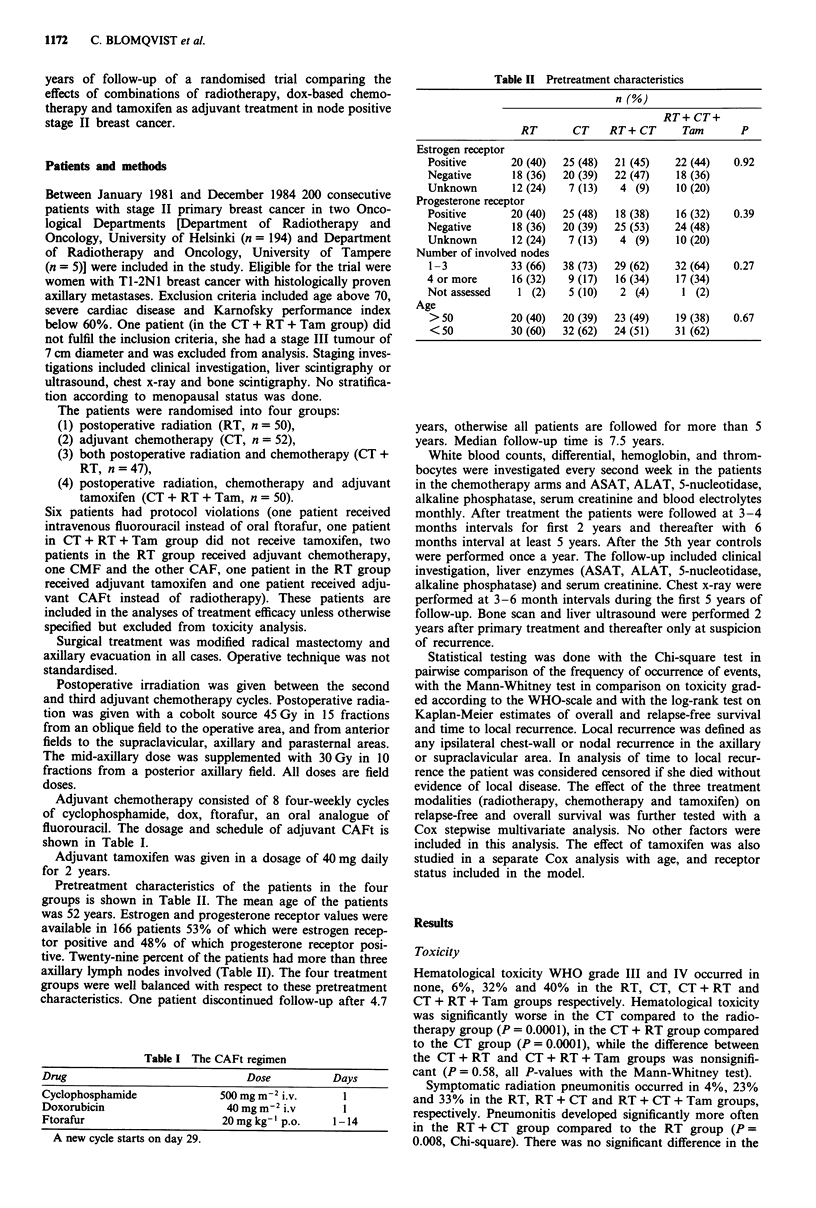
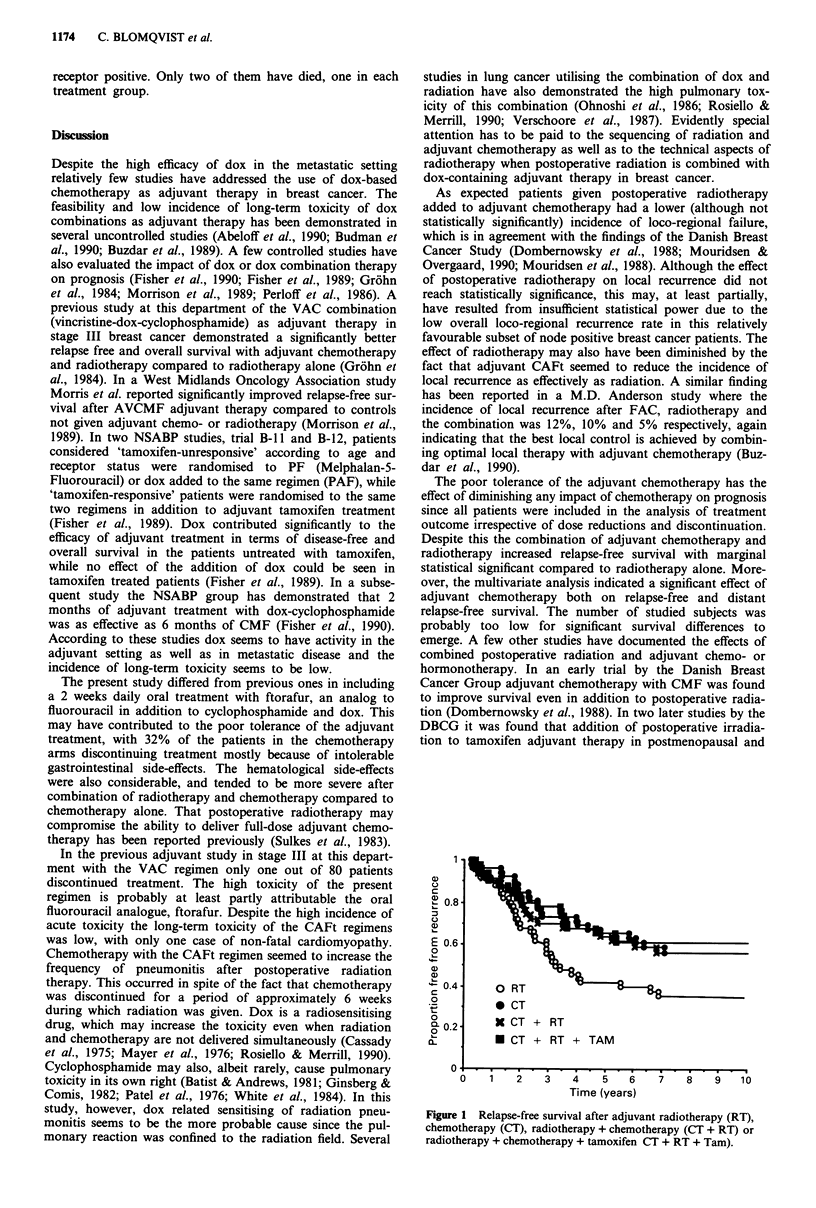
Two hundred patients with node positive stage II breast cancer were randomised to four groups after radical mastectomy and axillary evacuation: (1) Postoperative radiotherapy, (2) Adjuvant chemotherapy with eight courses of CAFt (cyclophosphamide 500 mg m-2 + doxorubicin 40 mg/m-2 + ftorafur 20 mg kg-1 orally day 1-14) every fourth week, (3) Postoperative radiotherapy and adjuvant chemotherapy and (4) postoperative radiation, adjuvant chemotherapy and tamoxifen 40 mg daily for 2 years. Thirty-two per cent of the patients discontinued treatment due to GI-toxicity, while 26% required dose reductions due to leukopenia. Radiation pneumonitis was more frequent after the combination of postoperative radiotherapy with chemotherapy. There was a better relapse-free survival in the groups receiving chemotherapy compared to radiotherapy alone (P = 0.05), which was highly significant in a multivariate Cox analysis (P = 0.004). No significant survival differences were seen. Tamoxifen had no clear overall effect but there were better relapse-free (P = 0.04) and overall (P = 0.004) survival with tamoxifen in estrogen receptor positive patients, while estrogen receptor negative patients had a somewhat poorer survival (P = 0.07) after tamoxifen. Local control was better (NS) after the combination (93%) radiotherapy and chemotherapy compared to either treatment alone (76% with radiotherapy and 74% with chemotherapy at 5 years).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeloff M. D., Beveridge R. A., Donehower R. C., Fetting J. H., Davidson N. E., Gordon G. G., Waterfield W. C., Damron D. J. Sixteen-week dose-intense chemotherapy in the adjuvant treatment of breast cancer. J Natl Cancer Inst. 1990 Apr 4;82(7):570–574. doi: 10.1093/jnci/82.7.570. [DOI] [PubMed] [Google Scholar]
- Batist G., Andrews J. L., Jr Pulmonary toxicity of antineoplastic drugs. JAMA. 1981 Sep 25;246(13):1449–1453. [PubMed] [Google Scholar]
- Bianco A. R., De Placido S., Gallo C., Pagliarulo C., Marinelli A., Petrella G., D'Istria M., Delrio G. Adjuvant therapy with tamoxifen in operable breast cancer. 10 year results of the Naples (GUN) study. Lancet. 1988 Nov 12;2(8620):1095–1099. [PubMed] [Google Scholar]
- Brincker H. Distant recurrence in breast cancer. Survival expectations and first choice of chemotherapy regimen. Acta Oncol. 1988;27(6A):729–732. doi: 10.3109/02841868809091776. [DOI] [PubMed] [Google Scholar]
- Budman D. R., Korzun A. H., Aisner J., Younger J., Silver R., Costanza M., Rice M. A., Wood W. A feasibility study of intensive CAF as outpatient adjuvant therapy for stage II breast cancer in a cooperative group: CALGB 8443. Cancer Invest. 1990;8(6):571–575. doi: 10.3109/07357909009018922. [DOI] [PubMed] [Google Scholar]
- Buzdar A. U., Kau S. W., Smith T. L., Hortobagyi G. N. Ten-year results of FAC adjuvant chemotherapy trial in breast cancer. Am J Clin Oncol. 1989 Apr;12(2):123–128. doi: 10.1097/00000421-198904000-00007. [DOI] [PubMed] [Google Scholar]
- Buzdar A. U., McNeese M. D., Hortobagyi G. N., Smith T. L., Kau S., Fraschini G., Hug V., Ellerbroek N., Holmes F. A., Ames F. Is chemotherapy effective in reducing the local failure rate in patients with operable breast cancer? Cancer. 1990 Feb 1;65(3):394–399. doi: 10.1002/1097-0142(19900201)65:3<394::aid-cncr2820650303>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Cassady J. R., Richter M. P., Piro A. J., Jaffe N. Radiation-adriamycin interactions: preliminary clinical observations. Cancer. 1975 Sep;36(3):946–949. doi: 10.1002/1097-0142(197509)36:3<946::aid-cncr2820360316>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Cuzick J., Stewart H., Peto R., Baum M., Fisher B., Host H., Lythgoe J. P., Ribeiro G., Scheurlen H., Wallgren A. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep. 1987 Jan;71(1):15–29. [PubMed] [Google Scholar]
- Dombernowsky P., Brincker H., Hansen M., Mouridsen H. T., Overgaard M., Panduro J., Rose C., Axelsson C. K., Andersen J., Andersen K. W. Adjuvant therapy of premenopausal and menopausal high-risk breast cancer patients. Present status of the Danish Breast Cancer Cooperative Group Trials 77-B and 82-B. Acta Oncol. 1988;27(6A):691–697. doi: 10.3109/02841868809091771. [DOI] [PubMed] [Google Scholar]
- Fisher B., Brown A. M., Dimitrov N. V., Poisson R., Redmond C., Margolese R. G., Bowman D., Wolmark N., Wickerham D. L., Kardinal C. G. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990 Sep;8(9):1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- Fisher B., Redmond C., Brown A., Fisher E. R., Wolmark N., Bowman D., Plotkin D., Wolter J., Bornstein R., Legault-Poisson S. Adjuvant chemotherapy with and without tamoxifen in the treatment of primary breast cancer: 5-year results from the National Surgical Adjuvant Breast and Bowel Project Trial. J Clin Oncol. 1986 Apr;4(4):459–471. doi: 10.1200/JCO.1986.4.4.459. [DOI] [PubMed] [Google Scholar]
- Fisher B., Redmond C., Brown A., Wolmark N., Wittliff J., Fisher E. R., Plotkin D., Bowman D., Sachs S., Wolter J. Treatment of primary breast cancer with chemotherapy and tamoxifen. N Engl J Med. 1981 Jul 2;305(1):1–6. doi: 10.1056/NEJM198107023050101. [DOI] [PubMed] [Google Scholar]
- Fisher B., Redmond C., Wickerham D. L., Bowman D., Schipper H., Wolmark N., Sass R., Fisher E. R., Jochimsen P., Legault-Poisson S. Doxorubicin-containing regimens for the treatment of stage II breast cancer: The National Surgical Adjuvant Breast and Bowel Project experience. J Clin Oncol. 1989 May;7(5):572–582. doi: 10.1200/JCO.1989.7.5.572. [DOI] [PubMed] [Google Scholar]
- Ginsberg S. J., Comis R. L. The pulmonary toxicity of antineoplastic agents. Semin Oncol. 1982 Mar;9(1):34–51. [PubMed] [Google Scholar]
- Gröhn P., Heinonen E., Klefström P., Tarkkanen J. Adjuvant postoperative radiotherapy, chemotherapy, and immunotherapy in stage III breast cancer. Cancer. 1984 Aug 15;54(4):670–674. doi: 10.1002/1097-0142(1984)54:4<670::aid-cncr2820540414>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Høst H., Brennhovd I. O. The effect of post-operative radiotherapy in breast cancer. Int J Radiat Oncol Biol Phys. 1977 Nov-Dec;2(11-12):1061–1067. doi: 10.1016/0360-3016(77)90110-9. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Gordon N. H., Hubay C. A., Pearson O. H. Assessment of tamoxifen as adjuvant therapy in stage II breast cancer: a long-term follow-up. J Lab Clin Med. 1987 Mar;109(3):300–307. [PubMed] [Google Scholar]
- Mauriac L., Durand M., Chauvergne J., Bonichon F., Avril A., Mage P., Dilhuydy M. H., Le Treut A., Wafflart J., Marée D. Adjuvant trial for stage II receptor-positive breast cancer: CMF vs. CMF + tamoxifen in a single centre. Breast Cancer Res Treat. 1988 May;11(2):179–186. doi: 10.1007/BF01805842. [DOI] [PubMed] [Google Scholar]
- Mayer E. G., Poulter C. A., Aristizabal S. A. Complications of irradiation related to apparent drug potentiation by adriamycin. Int J Radiat Oncol Biol Phys. 1976 Nov-Dec;1(11-12):1179–1188. doi: 10.1016/0360-3016(76)90091-2. [DOI] [PubMed] [Google Scholar]
- Morrison J. M., Howell A., Kelly K. A., Grieve R. J., Monypenny I. J., Walker R. A., Waterhouse J. A. West Midlands Oncology Association trials of adjuvant chemotherapy in operable breast cancer: results after a median follow-up of 7 years. I. Patients with involved axillary lymph nodes. Br J Cancer. 1989 Dec;60(6):911–918. doi: 10.1038/bjc.1989.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouridsen H. T., Rose C., Overgaard M., Dombernowsky P., Panduro J., Thorpe S., Rasmussen B. B., Blichert-Toft M., Andersen K. W. Adjuvant treatment of postmenopausal patients with high risk primary breast cancer. Results from the Danish adjuvant trials DBCG 77 C and DBCG 82 C. Acta Oncol. 1988;27(6A):699–705. doi: 10.3109/02841868809091772. [DOI] [PubMed] [Google Scholar]
- Ohnoshi T., Hiraki S., Kawahara S., Yamashita H., Yonei T., Ishii J., Egawa T., Kozuka A., Hiraki Y., Kimura I. Randomized trial comparing chemotherapy alone and chemotherapy plus chest irradiation in limited stage small cell lung cancer: a preliminary report. Jpn J Clin Oncol. 1986 Sep;16(3):271–277. [PubMed] [Google Scholar]
- Patel A. R., Shah P. C., Rhee H. L., Sassoon H., Rao K. P. Cyclophosphamide therapy and interstitial pulmonary fibrosis. Cancer. 1976 Oct;38(4):1542–1549. doi: 10.1002/1097-0142(197610)38:4<1542::aid-cncr2820380416>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Rosiello R. A., Merrill W. W. Radiation-induced lung injury. Clin Chest Med. 1990 Mar;11(1):65–71. [PubMed] [Google Scholar]
- Rutqvist L. E., Cedermark B., Glas U., Johansson H., Rotstein S., Skoog L., Somell A., Theve T., Wilking N., Askergren J. Randomized trial of adjuvant tamoxifen combined with postoperative radiation therapy or adjuvant chemotherapy in postmenopausal breast cancer. Cancer. 1990 Jul 1;66(1):89–96. doi: 10.1002/1097-0142(19900701)66:1<89::aid-cncr2820660117>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Senanayake F. Adjuvant hormonal chemotherapy in early breast cancer: early results from a controlled trial. Lancet. 1984 Nov 17;2(8412):1148–1149. doi: 10.1016/s0140-6736(84)91572-1. [DOI] [PubMed] [Google Scholar]
- Sulkes A., Brufman G., Rizel S., Weshler Z., Biran S., Fuks Z. The effect of postoperative radiotherapy on the feasibility of optimal dose adjuvant CMF chemotherapy in stage II breast carcinoma. Int J Radiat Oncol Biol Phys. 1983 Jan;9(1):17–21. doi: 10.1016/0360-3016(83)90202-x. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., 4th, Knuiman M. W., Sleeper L. A., Olson J. E., Tormey D. C., Gilchrist K. W., Falkson G., Rosenthal S. N., Carbone P. P., Cummings F. J. Six-year results of the Eastern Cooperative Oncology Group trial of observation versus CMFP versus CMFPT in postmenopausal patients with node-positive breast cancer. J Clin Oncol. 1989 Jul;7(7):879–889. doi: 10.1200/JCO.1989.7.7.879. [DOI] [PubMed] [Google Scholar]
- Tormey D. C., Gray R., Gilchrist K., Grage T., Carbone P. P., Wolter J., Woll J. E., Cummings F. J. Adjuvant chemohormonal therapy with cyclophosphamide, methotrexate, 5-fluorouracil, and prednisone (CMFP) or CMFP plus tamoxifen compared with CMF for premenopausal breast cancer patients. An Eastern Cooperative Oncology Group trial. Cancer. 1990 Jan 15;65(2):200–206. doi: 10.1002/1097-0142(19900115)65:2<200::aid-cncr2820650203>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Verschoore J., Lagrange J. L., Boublil J. L., Aubanel J. M., Blaive B., Pinto J., Namer M. Pulmonary toxicity of a combination of low-dose doxorubicin and irradiation for inoperable lung cancer. Radiother Oncol. 1987 Aug;9(4):281–288. doi: 10.1016/s0167-8140(87)80149-4. [DOI] [PubMed] [Google Scholar]
- White D. A., Orenstein M., Godwin T. A., Stover D. E. Chemotherapy-associated pulmonary toxic reactions during treatment for breast cancer. Arch Intern Med. 1984 May;144(5):953–956. [PubMed] [Google Scholar]


