Abstract
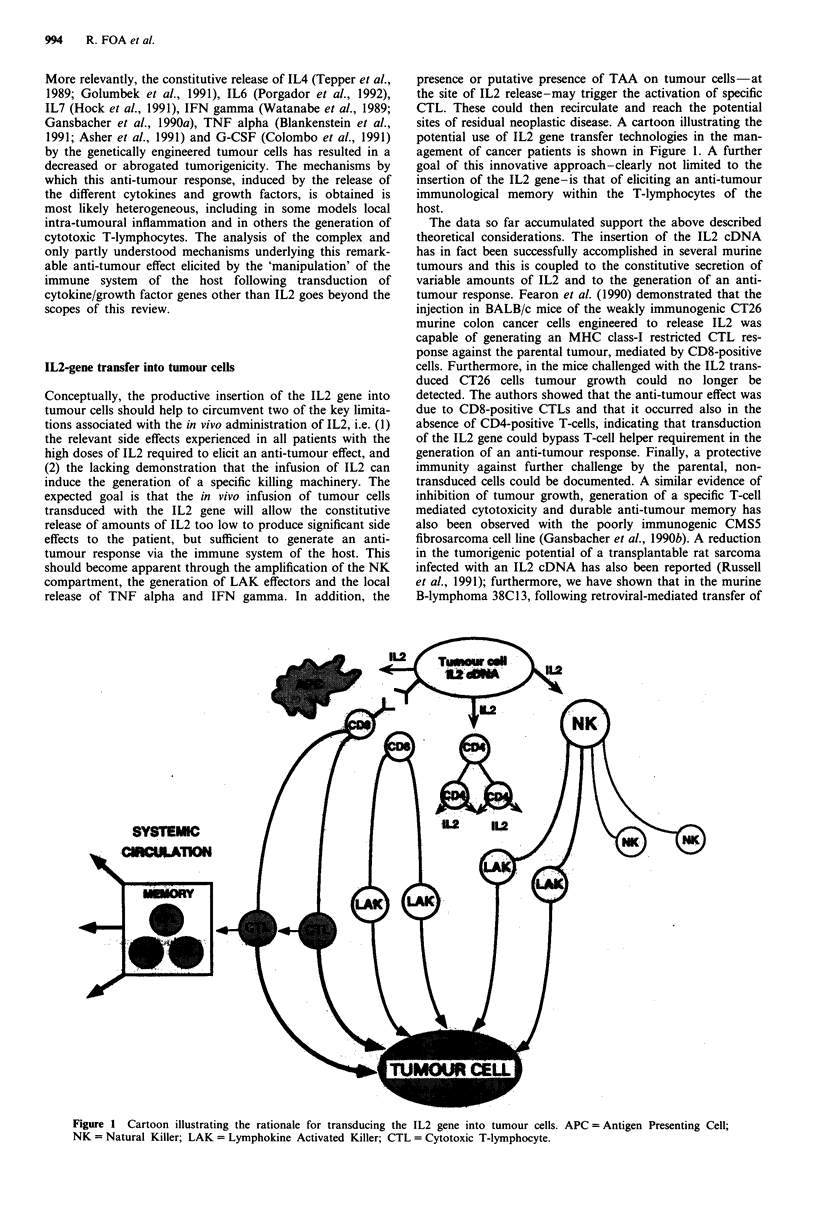
In this review we shall discuss the biological rationale and the clinical findings obtained using Interleukin 2 (IL2)-based immunotherapy in the management of cancer patients. Objective and long-lived clinical responses have been documented in a proportion of cases, particularly renal cell carcinoma, melanoma and acute myeloid leukaemia. Though encouraging, the clinical use of IL2 has so far been limited by toxicity, as well as by the heterogeneous and unpredictable responses and by the lack of specific anti-tumour effect. These considerations have led to the belief that more sophisticated technologies aimed at introducing the IL2 gene into the neoplastic cells may potentially overcome some of the limitations coupled to the in vivo infusion of high doses of IL2. The data accumulated in animal models and, more recently, also with human tumour cells indicate that the IL2 gene may be successfully inserted into neoplastic cells. The constitutive secretion of IL2 by the tumour cells leads to a reduced or abrogated tumorigenicity in several different tumour models. The evidence that in some experimental tumours the transduction of the IL2 gene into the neoplastic cells may elicit a specific cytotoxic response and confer anti-tumour memory, suggests that vaccination protocols based on this innovative strategy may represent a potential new tool in the management of cancer patients.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A., Chervenick P. A., Whiteside T. L., Lotzová E., Herberman R. B. Interleukin 2 induction of lymphokine-activated killer (LAK) activity in the peripheral blood and bone marrow of acute leukemia patients. I. Feasibility of LAK generation in adult patients with active disease and in remission. Blood. 1988 Mar;71(3):709–716. [PubMed] [Google Scholar]
- Anichini A., Fossati G., Parmiani G. Clonal analysis of cytotoxic T-lymphocyte response to autologous human metastatic melanoma. Int J Cancer. 1985 May 15;35(5):683–689. doi: 10.1002/ijc.2910350518. [DOI] [PubMed] [Google Scholar]
- Asher A. L., Mulé J. J., Kasid A., Restifo N. P., Salo J. C., Reichert C. M., Jaffe G., Fendly B., Kriegler M., Rosenberg S. A. Murine tumor cells transduced with the gene for tumor necrosis factor-alpha. Evidence for paracrine immune effects of tumor necrosis factor against tumors. J Immunol. 1991 May 1;146(9):3227–3234. [PMC free article] [PubMed] [Google Scholar]
- Blaise D., Olive D., Stoppa A. M., Viens P., Pourreau C., Lopez M., Attal M., Jasmin C., Monges G., Mawas C. Hematologic and immunologic effects of the systemic administration of recombinant interleukin-2 after autologous bone marrow transplantation. Blood. 1990 Sep 15;76(6):1092–1097. [PubMed] [Google Scholar]
- Blankenstein T., Qin Z. H., Uberla K., Müller W., Rosen H., Volk H. D., Diamantstein T. Tumor suppression after tumor cell-targeted tumor necrosis factor alpha gene transfer. J Exp Med. 1991 May 1;173(5):1047–1052. doi: 10.1084/jem.173.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri M. A., Murray C., Soiffer R. J., Klumpp T. R., Seiden M., Cochran K., Cameron C., Ish C., Buchanan L., Perillo D. Extended continuous infusion low-dose recombinant interleukin-2 in advanced cancer: prolonged immunomodulation without significant toxicity. J Clin Oncol. 1991 Dec;9(12):2110–2119. doi: 10.1200/JCO.1991.9.12.2110. [DOI] [PubMed] [Google Scholar]
- Chen W., Peace D. J., Rovira D. K., You S. G., Cheever M. A. T-cell immunity to the joining region of p210BCR-ABL protein. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1468–1472. doi: 10.1073/pnas.89.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M. P., Ferrari G., Stoppacciaro A., Parenza M., Rodolfo M., Mavilio F., Parmiani G. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med. 1991 Apr 1;173(4):889–897. doi: 10.1084/jem.173.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesina G., De Stefani A., Giovarelli M., Barioglio M. G., Cavallo G. P., Jemma C., Forni G. Treatment of recurrent squamous cell carcinoma of the head and neck with low doses of interleukin-2 injected perilymphatically. Cancer. 1988 Dec 15;62(12):2482–2485. doi: 10.1002/1097-0142(19881215)62:12<2482::aid-cncr2820621205>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Coulie P. G., Somville M., Lehmann F., Hainaut P., Brasseur F., Devos R., Boon T. Precursor frequency analysis of human cytolytic T lymphocytes directed against autologous melanoma cells. Int J Cancer. 1992 Jan 21;50(2):289–297. doi: 10.1002/ijc.2910500220. [DOI] [PubMed] [Google Scholar]
- Dawson M. M., Johnston D., Taylor G. M., Moore M. Lymphokine activated killing of fresh human leukaemias. Leuk Res. 1986;10(6):683–688. doi: 10.1016/0145-2126(86)90273-0. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Anderson W. F. Retroviral vectors for introduction of genes into mammalian cells. Biotechniques. 1988 Jul-Aug;6(7):608–614. [PubMed] [Google Scholar]
- Erard F., Corthesy P., Nabholz M., Lowenthal J. W., Zaech P., Plaetinck G., MacDonald H. R. Interleukin 2 is both necessary and sufficient for the growth and differentiation of lectin-stimulated cytolytic T lymphocyte precursors. J Immunol. 1985 Mar;134(3):1644–1652. [PubMed] [Google Scholar]
- Favrot M. C., Combaret V., Negrier S., Philip I., Thiesse P., Freydel C., Bijmann J. T., Franks C. R., Mercatello A., Philip T. Functional and immunophenotypic modifications induced by interleukin-2 did not predict response to therapy in patients with renal cell carcinoma. J Biol Response Mod. 1990 Apr;9(2):167–177. [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Fierro M. T., Liao X. S., Lusso P., Bonferroni M., Matera L., Cesano A., Lista P., Arione R., Forni G., Foa R. In vitro and in vivo susceptibility of human leukemic cells to lymphokine activated killer activity. Leukemia. 1988 Jan;2(1):50–54. [PubMed] [Google Scholar]
- Foa R., Caretto P., Fierro M. T., Bonferroni M., Cardona S., Guarini A., Lista P., Pegoraro L., Mandelli F., Forni G. Interleukin 2 does not promote the in vitro and in vivo proliferation and growth of human acute leukaemia cells of myeloid and lymphoid origin. Br J Haematol. 1990 May;75(1):34–40. doi: 10.1111/j.1365-2141.1990.tb02613.x. [DOI] [PubMed] [Google Scholar]
- Foa R., Fierro M. T., Cesano A., Guarini A., Bonferroni M., Raspadori D., Miniero R., Lauria F., Gavosto F. Defective lymphokine-activated killer cell generation and activity in acute leukemia patients with active disease. Blood. 1991 Aug 15;78(4):1041–1046. [PubMed] [Google Scholar]
- Foa R., Guarini A., Gillio Tos A., Cardona S., Fierro M. T., Meloni G., Tosti S., Mandelli F., Gavosto F. Peripheral blood and bone marrow immunophenotypic and functional modifications induced in acute leukemia patients treated with interleukin 2: evidence of in vivo lymphokine activated killer cell generation. Cancer Res. 1991 Feb 1;51(3):964–968. [PubMed] [Google Scholar]
- Foa R., Meloni G., Tosti S., Novarino A., Fenu S., Gavosto F., Mandelli F. Treatment of acute myeloid leukaemia patients with recombinant interleukin 2: a pilot study. Br J Haematol. 1991 Apr;77(4):491–496. doi: 10.1111/j.1365-2141.1991.tb08615.x. [DOI] [PubMed] [Google Scholar]
- Forni G., Fujiwara H., Martino F., Hamaoka T., Jemma C., Caretto P., Giovarelli M. Helper strategy in tumor immunology: expansion of helper lymphocytes and utilization of helper lymphokines for experimental and clinical immunotherapy. Cancer Metastasis Rev. 1988 Dec;7(4):289–309. doi: 10.1007/BF00051371. [DOI] [PubMed] [Google Scholar]
- Forni G., Giovarelli M., Santoni A. Lymphokine-activated tumor inhibition in vivo. I. The local administration of interleukin 2 triggers nonreactive lymphocytes from tumor-bearing mice to inhibit tumor growth. J Immunol. 1985 Feb;134(2):1305–1311. [PubMed] [Google Scholar]
- Gansbacher B., Bannerji R., Daniels B., Zier K., Cronin K., Gilboa E. Retroviral vector-mediated gamma-interferon gene transfer into tumor cells generates potent and long lasting antitumor immunity. Cancer Res. 1990 Dec 15;50(24):7820–7825. [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D., Laszlo J. Interferon therapy in cancer: from imaginon to interferon. Cancer Res. 1986 Sep;46(9):4315–4329. [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. J., Prentice H. G., Heslop H. E., Bello-Fernandez C., Bianchi A. C., Galazka A. R., Brenner M. K. Effects of recombinant interleukin-2 administration on cytotoxic function following high-dose chemo-radiotherapy for hematological malignancy. Blood. 1989 Nov 15;74(7):2335–2342. [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hank J. A., Kohler P. C., Weil-Hillman G., Rosenthal N., Moore K. H., Storer B., Minkoff D., Bradshaw J., Bechhofer R., Sondel P. M. In vivo induction of the lymphokine-activated killer phenomenon: interleukin 2-dependent human non-major histocompatibility complex-restricted cytotoxicity generated in vivo during administration of human recombinant interleukin 2. Cancer Res. 1988 Apr 1;48(7):1965–1971. [PubMed] [Google Scholar]
- Heslop H. E., Gottlieb D. J., Bianchi A. C., Meager A., Prentice H. G., Mehta A. B., Hoffbrand A. V., Brenner M. K. In vivo induction of gamma interferon and tumor necrosis factor by interleukin-2 infusion following intensive chemotherapy or autologous marrow transplantation. Blood. 1989 Sep;74(4):1374–1380. [PubMed] [Google Scholar]
- Higuchi C. M., Thompson J. A., Cox T., Lindgren C. G., Buckner C. D., Fefer A. Lymphokine-activated killer function following autologous bone marrow transplantation for refractory hematological malignancies. Cancer Res. 1989 Oct 15;49(20):5509–5513. [PubMed] [Google Scholar]
- Higuchi C. M., Thompson J. A., Petersen F. B., Buckner C. D., Fefer A. Toxicity and immunomodulatory effects of interleukin-2 after autologous bone marrow transplantation for hematologic malignancies. Blood. 1991 Jun 15;77(12):2561–2568. [PubMed] [Google Scholar]
- Hock H., Dorsch M., Diamantstein T., Blankenstein T. Interleukin 7 induces CD4+ T cell-dependent tumor rejection. J Exp Med. 1991 Dec 1;174(6):1291–1298. doi: 10.1084/jem.174.6.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere R., Rosenberg S. A. Successful immunotherapy of murine experimental hepatic metastases with lymphokine-activated killer cells and recombinant interleukin 2. Cancer Res. 1985 Aug;45(8):3735–3741. [PubMed] [Google Scholar]
- Ley V., Langlade-Demoyen P., Kourilsky P., Larsson-Sciard E. L. Interleukin 2-dependent activation of tumor-specific cytotoxic T lymphocytes in vivo. Eur J Immunol. 1991 Mar;21(3):851–854. doi: 10.1002/eji.1830210350. [DOI] [PubMed] [Google Scholar]
- Lim S. H., Newland A. C., Kelsey S., Bell A., Offerman E., Rist C., Gozzard D., Bareford D., Smith M. P., Goldstone A. H. Continuous intravenous infusion of high-dose recombinant interleukin-2 for acute myeloid leukaemia--a phase II study. Cancer Immunol Immunother. 1992;34(5):337–342. doi: 10.1007/BF01741555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lista P., Fierro M. T., Liao X. S., Bonferroni M., Brizzi M. F., Porcu P., Pegoraro L., Foa R. Lymphokine-activated killer (LAK) cells inhibit the clonogenic growth of human leukemic stem cells. Eur J Haematol. 1989 May;42(5):425–430. doi: 10.1111/j.1600-0609.1989.tb01465.x. [DOI] [PubMed] [Google Scholar]
- Lotzová E., Savary C. A., Totpal K., Schachner J., Lichtiger B., McCredie K. B., Freireich E. J. Highly oncolytic adherent lymphocytes: therapeutic relevance for leukemia. Leuk Res. 1991;15(4):245–254. doi: 10.1016/0145-2126(91)90127-f. [DOI] [PubMed] [Google Scholar]
- Maraninchi D., Blaise D., Viens P., Brandely M., Olive D., Lopez M., Sainty D., Marit G., Stoppa A. M., Reiffers J. High-dose recombinant interleukin-2 and acute myeloid leukemias in relapse. Blood. 1991 Nov 1;78(9):2182–2187. [PubMed] [Google Scholar]
- McLachlin J. R., Cornetta K., Eglitis M. A., Anderson W. F. Retroviral-mediated gene transfer. Prog Nucleic Acid Res Mol Biol. 1990;38:91–135. doi: 10.1016/s0079-6603(08)60709-6. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Schwarz S. L., Rosenberg S. A. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984 Sep 28;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A. T., Gerard J. P., Henderson L. E., Farrar W., Hopkins R. F., 3rd, Herberman R. B., Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984 Aug;133(2):779–783. [PubMed] [Google Scholar]
- Oshimi K., Oshimi Y., Akutsu M., Takei Y., Saito H., Okada M., Mizoguchi H. Cytotoxicity of interleukin 2-activated lymphocytes for leukemia and lymphoma cells. Blood. 1986 Oct;68(4):938–948. [PubMed] [Google Scholar]
- Parkinson D. R., Abrams J. S., Wiernik P. H., Rayner A. A., Margolin K. A., Van Echo D. A., Sznol M., Dutcher J. P., Aronson F. R., Doroshow J. H. Interleukin-2 therapy in patients with metastatic malignant melanoma: a phase II study. J Clin Oncol. 1990 Oct;8(10):1650–1656. doi: 10.1200/JCO.1990.8.10.1650. [DOI] [PubMed] [Google Scholar]
- Porgador A., Tzehoval E., Katz A., Vadai E., Revel M., Feldman M., Eisenbach L. Interleukin 6 gene transfection into Lewis lung carcinoma tumor cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992 Jul 1;52(13):3679–3686. [PubMed] [Google Scholar]
- Reittie J. E., Gottlieb D., Heslop H. E., Leger O., Drexler H. G., Hazlehurst G., Hoffbrand A. V., Prentice H. G., Brenner M. K. Endogenously generated activated killer cells circulate after autologous and allogeneic marrow transplantation but not after chemotherapy. Blood. 1989 Apr;73(5):1351–1358. [PubMed] [Google Scholar]
- Rosenberg S. A. Immunotherapy and gene therapy of cancer. Cancer Res. 1991 Sep 15;51(18 Suppl):5074s–5079s. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Aebersold P. M., Linehan W. M., Seipp C. A., White D. E. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Russell S. J., Eccles S. A., Flemming C. L., Johnson C. A., Collins M. K. Decreased tumorigenicity of a transplantable rat sarcoma following transfer and expression of an IL-2 cDNA. Int J Cancer. 1991 Jan 21;47(2):244–251. doi: 10.1002/ijc.2910470213. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Soiffer R. J., Murray C., Cochran K., Cameron C., Wang E., Schow P. W., Daley J. F., Ritz J. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood. 1992 Jan 15;79(2):517–526. [PubMed] [Google Scholar]
- Sondel P. M., Kohler P. C., Hank J. A., Moore K. H., Rosenthal N. S., Sosman J. A., Bechhofer R., Storer B. Clinical and immunological effects of recombinant interleukin 2 given by repetitive weekly cycles to patients with cancer. Cancer Res. 1988 May 1;48(9):2561–2567. [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Shulman K. L., Benyunes M. C., Lindgren C. G., Collins C., Lange P. H., Bush W. H., Jr, Benz L. A., Fefer A. Prolonged continuous intravenous infusion interleukin-2 and lymphokine-activated killer-cell therapy for metastatic renal cell carcinoma. J Clin Oncol. 1992 Jun;10(6):960–968. doi: 10.1200/JCO.1992.10.6.960. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Kuribayashi K., Miyatake S., Nishihara K., Nakayama E., Taniyama T., Sakata T. Exogenous expression of mouse interferon gamma cDNA in mouse neuroblastoma C1300 cells results in reduced tumorigenicity by augmented anti-tumor immunity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9456–9460. doi: 10.1073/pnas.86.23.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West W. H., Tauer K. W., Yannelli J. R., Marshall G. D., Orr D. W., Thurman G. B., Oldham R. K. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987 Apr 9;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- de Vries J. E., Spits H. Cloned human cytotoxic T lymphocyte (CTL) lines reactive with autologous melanoma cells. I. In vitro generation, isolation, and analysis to phenotype and specificity. J Immunol. 1984 Jan;132(1):510–519. [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]


