Abstract
The seasonal development of life-history traits is influenced by many environmental factors. The impact of photoperiodic and non-photoperiodic factors on nest building and egg laying has been rarely investigated in non-domesticated avian species for which long term field data sets are available. Former investigations showed that blue tits originating from geographically close populations in the Mediterranean region do not respond in the same way to photoperiodic factors in semi-natural outdoor conditions. Here we show experimentally that nest building and onset of egg laying in captive blue tits is also proximately influenced by non-photoperiodic factors, including aspects related to aviary characteristics and social interactions between birds of the two sexes originating from different local Mediterranean study populations. In two successive experiments, we show that 1) increasing the volume of the aviary advanced the egg laying period of one specific population by almost one month, and 2) crossing pairs of birds from different origins strongly reduced the nest building and egg laying behaviours. These results indicate that obtaining biologically relevant breeding results in captivity with wild birds requires the control and experimental manipulation of a wide array of complex environmental cues.
Keywords: avian reproduction, bird, captive animals, environmental factors, Parus caeruleus, seasonal breeding
1. Introduction
To adjust the timing of their reproduction with the annual peak of food availability, birds proximately respond to a specific set of environmental predictive cues (Murton and Westwood, 1977). In temperate zones, where the optimal breeding date is predictable across years, photoperiod is generally considered to be the key factor triggering the initiation of the seasonal sexual recrudescence of the gonads (Dawson et al., 2001; Sharp, 2005; Wingfield and Hunt, 2002). The action of photoperiod is then completed by a wide array of additional cues, the “supplementary information” (Wingfield and Moore, 1987; Wingfield and Kenagy, 1991) that are used to fine tune the seasonal activation of the hypothalamopituitary-gonadal (HPG) axis (Wingfield and Farner, 1993). Numerous studies have demonstrated the determinant role of ambient temperature, food abundance, previous reproductive experience, phenology of the vegetation, and social and stress factors on the determinism of the timing of reproduction (Breuner and Hahn, 2003; Grieco et al., 2002; Moore, 1982; 1983; Salvante and Williams, 2003; Visser and Lambrechts, 1999). The degree to which non-photoperiodic factors are integrated into the physiological control of the timing of breeding depends both of the species and the environments considered (Hau et al., 2000; Hau, 2001; Wingfield et al., 1992).
In the Mediterranean region, where habitats are highly contrasted, the optimal breeding time varies from one place to another, even at a micro-geographic scale (e.g. Blondel et al., 1987; 1999). In tits, the optimal time of reproduction is most often defined by the period when caterpillar abundance is maximal and this factor is supposed to play a key role in the ultimate control of the reproductive season (Perrins and McCleery, 1989; Perrins, 1991; Visser et al., 2004; but see Naef-Daenzer et al., 2001). Therefore deciduous habitats promote early egg laying dates, whereas evergreen habitats promote late egg laying dates (e.g. Blondel et al., 1987; 1993; Clamens and Isenmann, 1989). Different blue tit populations thus show large between-habitat variation of their egg laying dates and some populations breed at least one month earlier than others, despite the fact they are only 25 km apart.
Several experiments on captive birds have suggested that these differences in egg laying dates may have a genetic basis that would specifically mediate a differential response to photoperiodic cues. When blue tits originating either from a deciduous (Mediterranean-mainland) or an evergreen (Corsica-Pirio) oak woodland (same latitude and altitude) were settled in separate aviaries under natural photoperiods, the two populations maintained a significant difference in their respective timing of breeding, as predicted by corresponding field data (Lambrechts and Dias, 1993; Lambrechts et al., 1996; 1999). However, when these same populations were directly exposed to very long photoperiods (17L:7D), they bred simultaneously, suggesting that the two populations have different response threshold to photoperiodic cues, with birds originating from the evergreen forest having a higher response threshold to daylenght (Lambrechts et al., 1996; 1997). In contrast, another Corsican blue tit population (Corsica-Muro) originating from a similar deciduous forest as the Mainland population, showed much more phenotypic plasticity than the two other groups. This Muro population, which breeds early in the field, showed a two-month delay of egg laying dates when settled in aviaries under natural photoperiods, and bred even significantly later than the latest blue tit population originating from the evergreen habitat (Lambrechts et al., 1999). The exact reasons for this delayed breeding are unknown, but may be related to a different reaction to the artificial environment. As a consequence, the captive behaviour of this particular population demonstrates that photoperiod per se is not the only environmental factor that times the Mediterranean blue tit reproduction, but that other supplemental, non-photoperiodic, factors are also involved.
Supplemental factors, such as social cues exchanged between conspecifics, were recently suspected to play a decisive role in the control of the seasonal recrudescence of the reproductive system in male tits in early spring (Caro et al, 2005a; 2005b; 2006a). Despite the one month difference in egg laying period, two adjacent Corsican populations of male tits that live in early deciduous (Corsica-Muro) and late evergreen (Corsica-Pirio) forests, showed an almost simultaneous start of sexual development in late winter (Caro et al., 2006a). In contrast, female gonadal development remained highly asynchronous between these same populations, with females from the evergreen forest starting their follicle development almost one month later than in the deciduous population (Caro et al., submitted). It is only later during the pre-breeding season, in March, that the rates of development of males began to differ, with males from Corsica-Pirio showing a slower testis growth that the males from Corsica-Muro (Caro et al., 2005a, 2006a). Based on these observations, it was hypothesized that this slowing down of the male testis growth in the late evergreen population was caused by the lack of social sexual stimulation emanating from the non-receptive females during this early spring period. Similarly, the lack of specific cues or resources may lead to abnormal timing of breeding in aviaries (Lambrechts et al., 1999) or affects seasonal development of the HPG axis in the field (Caro et al., 2006a).
To test the specific roles of non-photoperiodic factors on breeding in captivity, we conducted two separate experiments. In one experiment, we created more natural breeding conditions in captivity, especially through a change in aviary size increasing opportunities to fly around and to escape more efficiently from perceived ground predators (e.g. humans visiting feeders) inside the aviaries. We expected more natural breeding dates in larger aviaries reflecting more natural conditions (cf. Lambrechts et al., 1999) than in smaller aviaries associated with more artificial conditions. In the second experiment, we investigated the potential role of male-female interactions in the expression of nest building and egg laying dates. Captive birds originating from different study populations in the wild were paired, and the breeding dates recorded. Lower breeding performance was especially predicted when pair members originated from populations expressing distinct breeding dates in the wild. The results indicate that the timing of nest building and egg laying are influenced proximately by a complex array of non-photoperiodic factors, in addition to the well established role of photoperiod.
2. Materials and methods
Pairs of wild blue tits from the Mediterranean region were placed in standardized conditions in large outdoor aviaries containing deciduous (Quercus humilis, Ficus carica) and/or evergreen (Quercus ilex, Ligustrum ovalifolium) vegetation. All birds were fed ad libitum with the same food and pairs were distributed randomly regarding their origin in similar aviaries that were located at the same latitude and altitude and exposed to similar lighting conditions (natural photoperiod). Pairs of birds were housed in adjacent aviaries visually isolated thanks to opaque canvas covers, in order to limit the potential influence of social information, exchanged between the different groups of captive birds, on the timing of breeding (i.e. Meijer and Langer, 1995). Acoustic contacts were however possible across aviaries. Blue tits originating from early breeding populations on the French Mediterranean mainland (Mediterranean-mainland, broad-leaved deciduous oak forest in the most part) and from a late breeding Corsican population (Corsica-Pirio, evergreen oak forest) were studied in captivity starting in 1986 (see Perret et al., 1989; Blondel et al., 1990; Lambrechts and Dias, 1993); blue tits from an early breeding Corsican population (Corsica-Muro, broad-leaved deciduous oak forest) were investigated starting in 1994 (Lambrechts et al., 1999; Lambrechts and Perret, 2000). All breeding data presented here were collected in birds kept under natural photoperiods. Only first clutches of eggs laid were considered. Birds born in captivity were excluded from the analyses, and only the first reproduction was considered in birds that bred during several successive years. In a preliminary set of statistical analyses, birds born in captivity and multiple reproductions of the same birds in aviaries were included to increase the sample sizes. The results of these analyses were almost identical to those presented in this manuscript, demonstrating that the conclusions presented here are robust and reliable. However, to avoid potential pseudo-replication in the analyses, and to present an easily repeatable set of experimental procedures, we finally preferred to exclude these birds in this presentation. The samples used in the present analyses include egg laying data used in former studies (see above) and additional breeding data collected during two different sets of experiments conducted between 2001 and 2005.
2.1. Experiment 1: effect of aviary characteristics
In previous studies, pairs of captive blue tits from the Mediterranean-mainland (early breeding) and Corsica-Pirio (late breeding) populations housed in 27 m3 aviaries (one pair per aviary) maintained a significant difference in their respective timing of breeding, as predicted by corresponding field data (Lambrechts and Dias, 1993; Lambrechts et al., 1996; 1999). Therefore, pairs originating from Corsica-Muro (early breeding population) were expected to show similar egg laying dates in aviaries as their mainland counterparts originating from the same type of habitat. However, these pairs showed significantly delayed laying dates in captivity; they bred almost two months later than in normal field conditions, and more than two weeks later than the late Corsica-Pirio birds raised in the same conditions (Lambrechts et al., 1999). Based on these abnormally late breeding dates in captivity, it was hypothesized that Corsica-Muro birds may have difficulties in coping with new and artificial environments. To increase the range of environmental cues available, we studied in 2001 and 2002 the breeding dates of 4 pairs of Corsica-Muro birds that were introduced separately in larger aviaries (3 pairs in 160 m3 and 1 pair in 320 m3 aviaries; all aviaries were 3 m high). Former investigations showed that the age (yearlings vs adults) at which birds were put in captivity does not have a significant impact on egg laying dates (cf. Lambrechts et al., 1999). In the present study, statistical analysis by two-way ANOVA (age and origin of pairs as factors) of the egg laying dates did not identified a significant overall effect of age (F1,16 = 1.396, P = 0.254), and the interaction between the two factors was not significant (F2,16 = 0.157, P = 0.856). This factor was therefore not considered in the analyses hereafter. We analyzed the nest building and egg laying success along with the respective egg laying dates of each of the following four groups: Mediterranean-mainland (further referred as Mainland; n = 7), Corsica-Muro (Muro; n = 26), Corsica-Muro in >160m3 aviaries (MuroL; n = 4), and Corsica-Pirio (Pirio; n = 14). Except the MuroL pairs, all other groups were housed in 27 m3 aviaries.
2.2. Experiment 2: role of social interactions
The initiation of late winter/early spring development of the brain song system and HPG axis in males is approximately simultaneous in the Corsican blue tit populations from Muro and Pirio, despite an average one-month difference in the egg laying dates (Caro et al., 2005a; 2005b; 2006a). Females, however, present a clearly differentiated (site-specific) gonadal development. Quantitative genetics indicate that the genetic contribution to the egg laying dates is sex-specific with females playing a much larger role than males (Caro et al., submitted; 2006b). Based on these results, it was hypothesized that females in these populations may drive both differential timing of egg-laying and male sexual development.
Therefore, in a second set of experiments in captivity performed during the spring in 2004 and 2005 (in the 27 m3 for all pairs except for 3 Pirio/Pirio pairs that were kept in the 160 m3 aviaries), we tested the specific role of females (and males) in the timing of egg laying. Since no statistical influence of the size of the aviaries (27m3 vs 160m3) could be detected on the number of Pirio/Pirio pairs that did built a nest (Fisher exact probability test: P = 0.339) or laid eggs (Fisher exact probability test: P = 0.255), and on the egg laying dates (One-way ANOVA – aviary size as factor – F1,2 = 0.021, P = 0.898), all available breeding data were combined.
Individual blue tits were trapped in the field (Mediterranean-mainland, Corsica-Pirio and Corsica-Muro populations) between mid-January and early February in 2004 and 2005, and three groups of pairs were established in aviaries as follows: one control group made of pairs of birds from ‘late’ Corsica-Pirio population (called Pirio/Pirio; n = 11), and two crossed groups where females from the ‘early’ mainland population were crossed with males from either ‘early’ Corsica-Muro (Muro/Mainland; n = 17) or ‘late’ Corsica-Pirio (Pirio/Mainland; n = 13) populations. If females control the timing of reproduction in Mediterranean blue tits, one would expect later nest building and laying dates in the control Pirio/Pirio group than in both crossed groups in which females come from the early breeding mainland population. In contrast, if males play a predominant role in the determination of the egg laying dates, we would expect significantly later laying dates in the Pirio/Pirio and Pirio/Mainland groups, than in the Muro/Mainland group.
The numbers of pairs engaging in nest building and egg laying were analyzed by Chi Square tests (or Fisher Exact Probability tests when sample sizes were small). One-way analyses of variance (ANOVA) were used to compare egg laying dates. Because individual and average laying dates in captivity are extremely stable from year to year, data from different years were combined (Lambrechts et al., 1996; 1997; 1999). Post hoc comparisons were carried out using Fisher Exact Probability tests (in Chi Square analyses) or Fisher Protected Least Significant Difference (PLSD) tests (in ANOVA analyses) in order to compare the experimental groups two by two. Effects were considered significant for P ≤ 0.05.
3. Results
3.1. Experiment 1: effect of aviary characteristics
In captivity, all four groups (Mainland, Muro, MuroL, Pirio) built nests efficiently (Fig. 1), with on average 82.3% of pairs demonstrating this behaviour. There was no significant difference in the numbers of pairs building nests between the four groups (χ2 = 6.223; P = 0.101). However, the proportion of pairs that laid eggs varied significantly between groups (χ2 = 17.518; P < 0.001). Fisher’s Exact Probability tests indicated that egg laying was rarer in the Muro than in the three other groups (see Fig. 1).
Fig. 1.
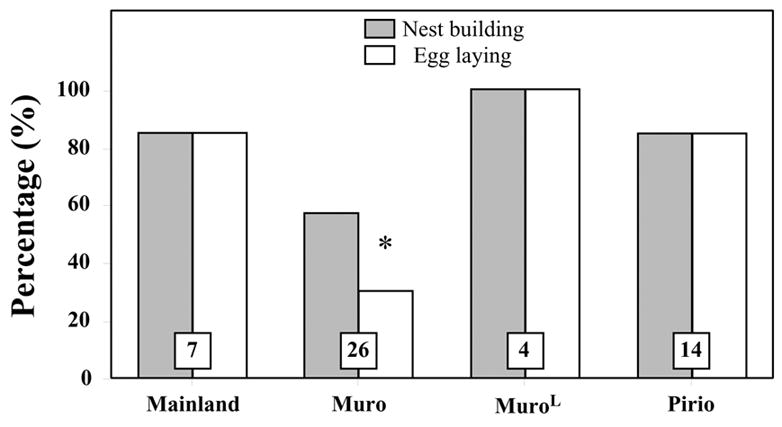
Proportion of blue tit pairs that built a nest and laid eggs in captivity between 1986 and 2003. Mainland, Muro and Pirio pairs were housed in 27 m3 aviaries, MuroL in >160 m3 aviaries. Total numbers of pairs used in this experiment are indicated in the corresponding bars. Results of post hoc comparisons are indicated at the top of the bars as follows: * P < 0.05 compared to the three other groups.
Within pairs that reached the egg laying stage, there was a strong effect of the group considered on the egg laying dates (F3,26 = 18.435, P < 0.0001). Post hoc tests (see symbols on Fig. 2A) showed that like in the field, the Mainland group laid significantly earlier than the Pirio group. The Muro group laid eggs significantly later than all the other groups but MuroL birds originating from the same population, when placed in large aviaries, laid eggs significantly earlier. These MuroL birds therefore displayed similar laying dates as their Mainland counterparts (Fig. 2A).
Fig. 2.
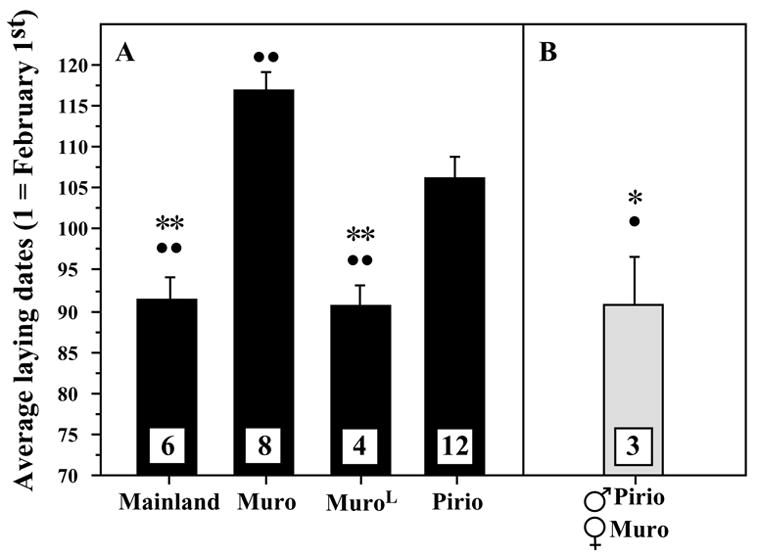
Onset of egg laying in captive blue tits (mean ± SE). All experimental groups were housed in 27 m3 aviaries, except the MuroL group that was kept in >160 m3 aviaries. Total numbers of pairs of birds used in this experiment are indicated in the corresponding bars. Laying dates are expressed with February 1stconsidered as day 1. Results of post hoc comparisons are indicated at the top of the bars as follows: Fig. 2A: ●● P < 0.01 compared to the Pirio group, ** P < 0.0001 compared to the Muro group; Fig. 2B: ● P < 0.01 compared to the Pirio group in 2A, * P < 0.0001 compared to the Muro group in 2A.
These results therefore indicate that the Corsica-Muro pairs housed in “small” aviaries reproduce less and later than in the field, but that this phenomenon disappears when they are housed in larger aviaries that are more similar to the natural conditions. Overall, the captive Mainland, MuroL and Pirio groups presented the same breeding order as in the field.
Furthermore, comparison with additional data coming from three mixed pairs (Pirio/Muro; see Fig. 2B) that were studied as part of another project not described here in detail, suggests that the late timing of breeding in the Muro group may have been induced by the males specifically. Indeed, three pairs of blue tits which included a female from Muro associated with a male from Pirio (Muro/Pirio group) and were housed in 27 m3 aviaries, laid eggs significantly earlier than the Pirio and Muro groups, but at similar dates as the MuroL pairs (Muro/Pirio vs MuroL vs Muro vs Pirio: F3,23 = 14.304, P < 0.0001) (see post hoc comparisons on Fig. 2B).]
Because early egg laying dates were observed in Muro birds housed in the “small” aviaries when the Muro male was replaced by another male from Pirio, we hypothesized that 1) the late laying date of the Muro pairs in “small” aviaries was induced by a sex-specific phenomenon concerning males preferentially, and that 2) when disruptive factors are minimized (by increasing the size of the aviary or replacing the male who is most affected by captivity), the laying dates were proximately determined by the origin of the female. To test this second hypothesis and assess the role of each sex on egg laying dates, we conducted a second experiment where birds from different origins were crossed in mixed pairs.
3.2. Experiment 2: role of social interactions
During this experiment, very large differences were observed between experimental groups in the percentage of pairs engaging in nest building and egg laying (Fig. 3). Fisher’s Exact Probability tests indicated that a significantly larger number of control Pirio/Pirio pairs started building nests compared to the two other groups (Pirio/Pirio vs Pirio/Mainland, P = 0.007; Pirio/Pirio vs Muro/Mainland, P = 0.002 ; see also symbols on Fig. 3). Control Pirio/Pirio birds also reached the egg laying stage significantly more often than the Muro/Mainland (P = 0.016), but not compared to the Pirio/Mainland (P = 0.101) (Fig. 3). Given that only 5 pairs laid eggs (4 in the Pirio/Pirio group; 1 in the Pirio/Mainland group, and none in the Muro/Mainland group), statistical analyses on the respective egg laying dates were meaningless and therefore not performed.
Fig. 3.
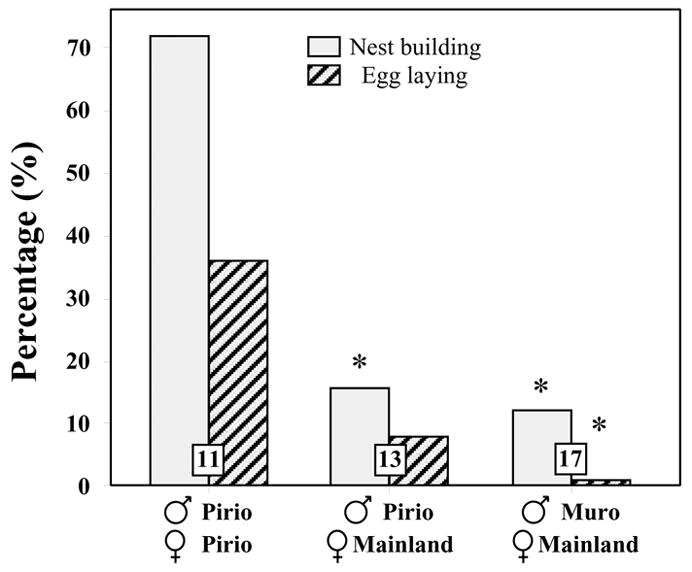
Proportion of blue tit pairs that built nest and laid eggs in captivity in 2004 and 2005. Total numbers of pairs of birds used in this experiment are indicated in the corresponding bars. Results of post hoc comparisons are indicated at the top of the bars as follows: * P < 0.05 compared to the control Pirio/Pirio group.
4. Discussion
Experiments with captive animals in standardized conditions offer the opportunity to test the relative influence of different environmental cues and resources on the expression of reproductive behaviours (Visser and Lambrechts, 1999). However, the reduced array of environmental factors available in captivity, including the drastic reduction in available space, can also lead to a reduced or abnormal spectrum of reproductive traits (Lambrechts et al., 1999; Perfito et al., 2005; Wingfield, 1983; Wingfield and Moore, 1987). This is particularly the case with non-domesticated wild species. For instance, in aviary experiments with wild species, males often show a complete development of the testis, but the circulating levels of sex steroids and gonadotropins are lower than in normal field conditions (Wingfield, 1984; Wingfield and Moore, 1987) and in females, full yolk deposition and egg laying are rarely observed (Wingfield, 1983). The data presented here indicate that supplementary factors play a significant role in the complete sequence of reproductive behaviours in Corsican blue tits maintained in aviaries, specifically in some local populations. They also suggest that one sex can be more affected by captivity than the other.
Previous work with Mediterranean blue tits has shown that large between-population differences, which do not exist in the wild (e.g. between Mainland and Muro birds), may appear in captive animals exposed to standardized conditions (Lambrechts et al., 1999). In particular, the blue tits from Muro may have more difficulties to cope with new and artificial environments than birds from Pirio, and this phenomenon may delay the reproductive behaviours observed in the “small” aviaries. The present study indicates that by increasing the space and consequently the array of environmental information available in captivity, birds may recover their population-typical life history trait responses. Captive birds originating from the same population (Muro) but placed in less restricted environmental conditions (e.g. aviaries with a much larger volume and a mixture of different vegetation types), showed during the present study a significant increase in the proportion of pairs laying eggs (Fig. 1) and a significant advancement in time of egg laying (Fig. 2A). Pairs of Muro birds held in the larger aviaries had the highest egg laying rates of all groups tested (Fig. 1) and their laying dates were similar to those observed in the field. Moreover, the fact that Muro females paired with a male from Pirio in “small” aviaries, laid significantly earlier than when they were kept with males from their own population (Fig. 2B), suggests that multiple independent modifications can result in similar improvements of the captive breeding physiology such as a change in the physical (cage size) or in the social (type of male mate) environment. These data are also consistent with the idea that, at the proximal level, the laying dates are predominantly determined by the origin of the female.
The exact mechanisms responsible for impaired and delayed reproduction of the Muro pairs housed in small aviaries, compared to their breeding behaviour observed in the field or in larger aviaries, are unknown; but these mechanisms are probably specific to this single population. Mainland and Pirio pairs housed in these small aviaries indeed reproduced very well (see Fig. 1) and maintained a significant between-population difference in the egg laying dates (Fig. 2A) as it is observed in the field, with Mainland birds originating from early deciduous habitat reproducing earlier than the Pirio birds originating from the late evergreen habitat. Furthermore, Pirio egg laying dates did not seem to be affected by the change in cage size (see §2.2 in the methods). The specific behaviour of the Muro birds may originate from fast local microevolutionary processes in combination with low dispersal on the island of Corsica that resulted in different genetic blue tit lines (Lambrechts et al., 1999). These different genetic blue tit lines may have evolved specific adaptation mechanisms to their local breeding habitats, using different environmental cues or using the same cues but at different thresholds to match the local optimal breeding time. Some specific environmental cues used by the Muro birds may either never reach their critical threshold, or even be totally absent in the small aviaries, causing impairments and delays in the timing of breeding. The two different types of aviaries do not only differ in their size, but also in the abundance of the vegetation, and, to some extend, by their vegetation diversity. As a consequence, the larger aviaries offer more opportunities to assess the phenology of the vegetation, to find natural food preys, to escape potential ground predators, and even to escape potential aggressiveness of the partner. All these non-mutually exclusive factors are potential causes of the impaired/delayed reproduction of the Muro birds in small aviaries.
Many experiments with captive subjects have previously been performed to test the influence of social factors on the endocrine physiology and reproduction of avian species (reviews in Ball and Balthazart, 2002; Wingfield et al., 1994). For example, male sparrows and cowbirds show increases in LH and testosterone plasma concentrations if exposed to a receptive female implanted with 17β-estradiol, a sex steroid that activates sexual behaviour in the female (Dufty and Wingfield, 1986; Moore, 1983; Wingfield and Moore, 1987). Conversely, complex male vocalizations accelerate female nest building behaviour and advance egg laying dates in canaries (Hinde and Steel, 1976; Kroodsma, 1976). In the present study, reproduction in captive Mediterranean blue tits was strongly hampered / inhibited in mixed pairs of males and females originating from different populations. Egg laying occurred so rarely that analysis of egg laying dates did not allow to test the hypothesis of a sex-specific control of the timing of reproduction. However this experiment clearly suggests that social signals exchanged between the partners are crucial for successful reproduction in this species. The exact nature of the social factors involved is still unknown, but a reproductive genetic isolation of the two sub-species (Mainland: Parus caeruleus caeruleus; Corsica: Parus caeruleus ogliostrae) can be excluded since fertilized eggs were laid (this study, unpubl. data). Furthermore, as mentioned above, results also suggest that the lack of proper environmental stimuli may have mainly affected a single sex. If males indeed have more difficulties in coping with captive conditions than females, one could then argue that contrary to the results expected, the lack of reproduction may have been driven by a disruption specific to males.
The present study demonstrates that the ability to cope with artificial environments may present large inter-populational differences. It seems also obvious that some life-history traits involved in the reproduction of Mediterranean blue tits may vary between the sexes. This was first demonstrated by recent field studies comparing the sexual development of males and females during the spring (Caro et al., 2006a; b; Caro et al., submitted) and exploring the genetic contribution of both sexes in the determination of the egg laying dates (subm.), and this is also suggested by the present study on captive birds (Fig. 2B). Studies of wild bird species investigating relevant physiological and behavioural responses to specific environmental factors should thus always be simultaneously conducted in the field and in captivity, with special attention paid to the sex considered, in order to avoid misinterpretations of the results.
Acknowledgments
These studies were supported by grants from the NINDS (NS 35467) and the Belgian FRFC (2.4562.05) to J.B. and from the Belgian FNRS (Crédits de mission) and the University of Liège (Bourse Patrimoine) to S.P.C. M.M.L. received financial support from the European Commission and the CNRS. S.P.C. is a FRIA Grant recipient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ball GF, Balthazart J. Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 2. Academic Press; San Diego: 2002. pp. 649–798. [Google Scholar]
- Blondel J, Clamens A, Cramm P, Gaubert H, Isenmann P. Population studies on tits in the Mediterranean region. Ardea. 1987;75:21–34. [Google Scholar]
- Blondel J, Dias PC, Maistre M, Perret P. Habitat heterogeneity and life-history variation of mediterranean blue tits (Parus caeruleus) Auk. 1993;110:511–520. [Google Scholar]
- Blondel J, Dias PC, Perret P, Maistre M, Lambrechts MM. Selection-based biodiversity at a small spatial scale in a low-dispersing insular bird. Science. 1999;285:1399–1402. doi: 10.1126/science.285.5432.1399. [DOI] [PubMed] [Google Scholar]
- Blondel J, Perret P, Maistre M. On the genetical basis of the laying-date in an island population of blue tits. J Evol Biol. 1990;3:469–475. [Google Scholar]
- Breuner CW, Hahn TP. Integrating stress physiology, environmental change, and behavior in free-living sparrows. Horm Behav. 2003;43:115–123. doi: 10.1016/s0018-506x(02)00020-x. [DOI] [PubMed] [Google Scholar]
- Caro SP, Balthazart J, Thomas DW, Lacroix A, Chastel O, Lambrechts MM. Endocrine correlates of the breeding asynchrony between two corsican populations of blue tits (Parus caeruleus) Gen Comp Endocrinol. 2005a;140:52–60. doi: 10.1016/j.ygcen.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Caro SP, Lambrechts MM, Balthazart J. Early seasonal development of brain song control nuclei in male blue tits. Neurosci Lett. 2005b;386:139–144. doi: 10.1016/j.neulet.2005.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro SP, Lambrechts MM, Chastel O, Sharp PJ, Thomas DW, Balthazart J. Simultaneous pituitary-gonadal recrudescence in two Corsican populations of male blue tits with asynchronous breeding dates. Horm Behav. 2006a;50:347–360. doi: 10.1016/j.yhbeh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Caro SP, Balthazart J, Lambrechts MM. The proximate basis of adaptive micro-geographic variation in reproductive phenology in male and female Blue Tits. J Ornithol. 2006b;147(Suppl 1):47–48. [Google Scholar]
- Clamens A, Isenmann P. Effect of supplemental food on the breeding of blue and great tits in Mediterranean habitats. Ornis Scan. 1989;20:36–42. [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Dufty AM, Wingfield JC. The influence of social cues on the reproductive endocrinology of male brown-headed cowbirds: field and laboratory studies. Horm Behav. 1986;20:222–234. doi: 10.1016/0018-506x(86)90020-6. [DOI] [PubMed] [Google Scholar]
- Grieco F, van Noordwijk AJ, Visser ME. Evidence for the effect of learning on timing of reproduction in blue tits. Science. 2002;296:136–138. doi: 10.1126/science.1068287. [DOI] [PubMed] [Google Scholar]
- Hau M, Wikelski M, Wingfield JC. Visual and nutritional food cues fine-tune timing of reproduction in a neotropical rainforest bird. J Exp Zool. 2000;286:494–504. [PubMed] [Google Scholar]
- Hau M. Timing of breeding in variable environments: Tropical birds as model systems. Horm Behav. 2001;40:281–290. doi: 10.1006/hbeh.2001.1673. [DOI] [PubMed] [Google Scholar]
- Hinde RA, Steel E. The effect of male song on an estrogen-dependent behavior pattern in the female canary (Serinus canarius) Horm Behav. 1976;7:293–304. doi: 10.1016/0018-506x(76)90035-0. [DOI] [PubMed] [Google Scholar]
- Kroodsma DE. Reproductive development in a female songbird: differential stimulation by quality of male song. Science. 1976;192:574–575. doi: 10.1126/science.192.4239.574. [DOI] [PubMed] [Google Scholar]
- Lambrechts MM, Dias PC. Differences in the onset of laying between island and mainland Mediterranean Blue Tits Parus caeruleus: phenotypic plasticity or genetic differences? Ibis. 1993;135:451–455. [Google Scholar]
- Lambrechts MM, Perret P, Blondel J. Adaptive differences in the timing of egg laying between different populations of birds result from variation in photoresponsiveness. Proc R Soc Lond B. 1996;263:19–22. [Google Scholar]
- Lambrechts MM, Blondel J, Maistre M, Perret P. A single response mechanism is responsible for evolutionary adaptive variation in a bird's laying date. Proc Nat Acad Sci USA. 1997;94:5153–5155. doi: 10.1073/pnas.94.10.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts MM, Perret P, Maistre M, Blondel J. Do experiments with captive non-domesticated animals make sense without population field studies? A case study with blue tit's breeding time. Proc R Soc Lond B. 1999;266:1311–1315. [Google Scholar]
- Lambrechts MM, Perret P. A long photoperiod overrides non-photoperiodic factors in blue tits' timing of reproduction. Proc R Soc Lond B. 2000;267:585–588. doi: 10.1098/rspb.2000.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer T, Langer U. Food availability and egg-laying of captive European starlings. Condor. 1995;97:718–728. [Google Scholar]
- Moore MC. Hormonal response of free-living male white-crowned sparrows to experimental manipulation of female sexual behavior. Horm Behav. 1982;16:323–329. doi: 10.1016/0018-506x(82)90030-7. [DOI] [PubMed] [Google Scholar]
- Moore MC. Effect of female displays on the endocrine physiology and behaviour of male White-crowned sparrows, Zonotrichia leucophrys. J Zool Lond. 1983;199:137–148. [Google Scholar]
- Murton RK, Westwood NJ. Avian breeding cycles. Oxford University Press; London: 1977. [Google Scholar]
- Naef-Daenzer L, Widmer F, Nuber M. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J Anim Ecol. 2001;70:730–738. [Google Scholar]
- Perfito N, Meddle SL, Tramontin AD, Sharp PJ, Wingfield JC. Seasonal gonadal recrudescence in song sparrows: Response to temperature cues. Gen Comp Endocrinol. 2005;143:121–128. doi: 10.1016/j.ygcen.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Perret P, Blondel J, Dervieux A, Maistre M, Colomb B. Composante génétique de la date de ponte chez la Mésange bleue Parus caeruleus L (Aves) C R Acad Sci Paris. 1989;308:527–530. [Google Scholar]
- Perrins CM, McCleery RH. Laying dates and clutch size in the great tit. Wilson Bull. 1989;101:236–253. [Google Scholar]
- Perrins CM. Tits and their caterpillar food supply. Ibis. 1991;133:49–54. [Google Scholar]
- Salvante KG, Williams TD. Effects of corticosterone on the proportion of breeding females, reproductive output and yolk precursor levels. Gen Comp Endocrinol. 2003;130:205–214. doi: 10.1016/s0016-6480(02)00637-8. [DOI] [PubMed] [Google Scholar]
- Sharp PJ. Photoperiodic regulation of seasonal breeding in birds. Ann NY Acad Sci. 2005;1040:189–199. doi: 10.1196/annals.1327.024. [DOI] [PubMed] [Google Scholar]
- Visser ME, Both C, Lambrechts MM. Global climate change leads to mistimed avian reproduction. Adv Ecol Res. 2004;35:89–110. [Google Scholar]
- Visser ME, Lambrechts MM. Information constraints in the timing of reproduction in temperate zone birds: Great and Blue Tits. In: Adams NJ, Slotow RH, editors. Proc 22 Int Ornithol Congr; Durban, BirdLife South Africa, Johannesburg. 1999. pp. 249–264. [Google Scholar]
- Wingfield JC, Farner DS. Endocrinology of reproduction in wild species. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. IX. Academic Press; New York: 1993. pp. 163–327. [Google Scholar]
- Wingfield JC. Environmental and endocrine control of avian reproduction: an ecological approach. In: Mikami S, Homma K, Wada H, editors. Avian endocrinology: environmental and ecological perspectives. Japan scientific societies press; Tokyo: 1983. pp. 265–288. [Google Scholar]
- Wingfield JC. Environmental and endocrine control of reproduction in the song sparrow, Melospiza melodia. I Temporal organization of the breeding cycle. Gen Comp Endocrinol. 1984;56:406–416. doi: 10.1016/0016-6480(84)90083-2. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Moore MC. Hormonal, social, and environmental factors in the reproductive biology of free-living male birds. In: Crews D, editor. Psychology of Reproduction: An evolutionary perspective. Prentice-Hall: Inc. Englewood Cliffs, New Jersey; 1987. pp. 148–175. [Google Scholar]
- Wingfield JC, Kenagy GJ. Natural regulation of reproductive cycles. In: Pawg PKT, Schreibruan MP, editors. Vertebrate endocrinology: fundamentals and biomedical implications. Academic Press Inc; San Diego: 1991. pp. 181–241. [Google Scholar]
- Wingfield JC, Hahn TP, Levin R, Honey P. Environmental predictability and control of gonadal cycles in birds. J Exp Zool. 1992;261:214–231. [Google Scholar]
- Wingfield JC, Whaling CS, Marler P. Communication in vertebrate aggression and reproduction: the role of hormones. In: Knobil E, Neill JD, editors. The physiology of reproduction. 2. Raven Press; New York: 1994. pp. 303–341. [Google Scholar]
- Wingfield JC, Hunt KE. Arctic spring: hormone-behavior interactions in a severe environment. Comp Biochem Physiol B-Biochem Mol Biol. 2002;132:275–286. doi: 10.1016/s1096-4959(01)00540-1. [DOI] [PubMed] [Google Scholar]


